Fibro-adipogenic progenitors of dystrophic mice are insensitive to NOTCH regulation of adipogenesis
- PMID: 31239312
- PMCID: PMC6599969
- DOI: 10.26508/lsa.201900437
Fibro-adipogenic progenitors of dystrophic mice are insensitive to NOTCH regulation of adipogenesis
Abstract
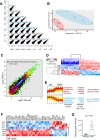
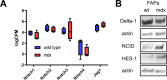
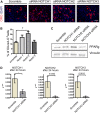
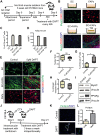
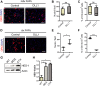
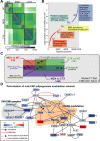
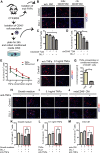
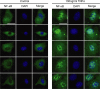
Fibro-adipogenic progenitors (FAPs) promote satellite cell differentiation in adult skeletal muscle regeneration. However, in pathological conditions, FAPs are responsible for fibrosis and fatty infiltrations. Here we show that the NOTCH pathway negatively modulates FAP differentiation both in vitro and in vivo. However, FAPs isolated from young dystrophin-deficient mdx mice are insensitive to this control mechanism. An unbiased mass spectrometry-based proteomic analysis of FAPs from muscles of wild-type and mdx mice suggested that the synergistic cooperation between NOTCH and inflammatory signals controls FAP differentiation. Remarkably, we demonstrated that factors released by hematopoietic cells restore the sensitivity to NOTCH adipogenic inhibition in mdx FAPs. These results offer a basis for rationalizing pathological ectopic fat infiltrations in skeletal muscle and may suggest new therapeutic strategies to mitigate the detrimental effects of fat depositions in muscles of dystrophic patients.
© 2019 Marinkovic et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Expression profile and overexpression outcome indicate a role for βKlotho in skeletal muscle fibro/adipogenesis.FEBS J. 2016 May;283(9):1653-68. doi: 10.1111/febs.13682. Epub 2016 Apr 13. FEBS J. 2016. PMID: 26881702 Free PMC article.
-
Metabolic reprogramming of fibro/adipogenic progenitors facilitates muscle regeneration.Life Sci Alliance. 2020 Feb 4;3(3):e202000646. doi: 10.26508/lsa.202000660. Print 2020 Mar. Life Sci Alliance. 2020. PMID: 32019766 Free PMC article.
-
Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice.EMBO Mol Med. 2013 Apr;5(4):626-39. doi: 10.1002/emmm.201202096. Epub 2013 Mar 18. EMBO Mol Med. 2013. PMID: 23505062 Free PMC article.
-
Fibro-adipogenic progenitors in physiological adipogenesis and intermuscular adipose tissue remodeling.Mol Aspects Med. 2024 Jun;97:101277. doi: 10.1016/j.mam.2024.101277. Epub 2024 May 23. Mol Aspects Med. 2024. PMID: 38788527 Free PMC article. Review.
-
Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease.FEBS J. 2022 Nov;289(21):6484-6517. doi: 10.1111/febs.16080. Epub 2021 Jul 6. FEBS J. 2022. PMID: 34143565 Review.
Cited by
-
Adipogenic-Myogenic Signaling in Engineered Human Muscle Grafts used to Treat Volumetric Muscle Loss.Adv Biol (Weinh). 2024 Dec;8(12):e2400113. doi: 10.1002/adbi.202400113. Epub 2024 Sep 18. Adv Biol (Weinh). 2024. PMID: 39294862
-
Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis.J Cachexia Sarcopenia Muscle. 2021 Jun;12(3):746-768. doi: 10.1002/jcsm.12700. Epub 2021 May 6. J Cachexia Sarcopenia Muscle. 2021. PMID: 33955709 Free PMC article.
-
Role of fibro-adipogenic progenitor cells in muscle atrophy and musculoskeletal diseases.Curr Opin Pharmacol. 2021 Jun;58:1-7. doi: 10.1016/j.coph.2021.03.003. Epub 2021 Apr 8. Curr Opin Pharmacol. 2021. PMID: 33839480 Free PMC article. Review.
-
Characterization of cellular senescence in aging skeletal muscle.Nat Aging. 2022 Jul;2(7):601-615. doi: 10.1038/s43587-022-00250-8. Epub 2022 Jul 15. Nat Aging. 2022. PMID: 36147777 Free PMC article.
-
The Notch signaling pathway in skeletal muscle health and disease.Muscle Nerve. 2022 Nov;66(5):530-544. doi: 10.1002/mus.27684. Epub 2022 Aug 15. Muscle Nerve. 2022. PMID: 35968817 Free PMC article. Review.
References
-
- Amir el AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe’er D (2013) viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol 31: 545–552. 10.1038/nbt.2594 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
