Vibrio cholerae filamentation promotes chitin surface attachment at the expense of competition in biofilms
- PMID: 31239347
- PMCID: PMC6628660
- DOI: 10.1073/pnas.1819016116
Vibrio cholerae filamentation promotes chitin surface attachment at the expense of competition in biofilms
Abstract
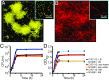
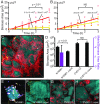
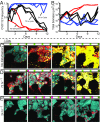
Collective behavior in spatially structured groups, or biofilms, is the norm among microbes in their natural environments. Though biofilm formation has been studied for decades, tracing the mechanistic and ecological links between individual cell morphologies and the emergent features of cell groups is still in its infancy. Here we use single-cell-resolution confocal microscopy to explore biofilms of the human pathogen Vibrio cholerae in conditions mimicking its marine habitat. Prior reports have noted the occurrence of cellular filamentation in V. cholerae, with variable propensity to filament among both toxigenic and nontoxigenic strains. Using a filamenting strain of V. cholerae O139, we show that cells with this morphotype gain a profound competitive advantage in colonizing and spreading on particles of chitin, the material many marine Vibrio species depend on for growth in seawater. Furthermore, filamentous cells can produce biofilms that are independent of primary secreted components of the V. cholerae biofilm matrix; instead, filamentous biofilm architectural strength appears to derive at least in part from the entangled mesh of cells themselves. The advantage gained by filamentous cells in early chitin colonization and growth is countered in long-term competition experiments with matrix-secreting V. cholerae variants, whose densely packed biofilm structures displace competitors from surfaces. Overall, our results reveal an alternative mode of biofilm architecture that is dependent on filamentous cell morphology and advantageous in environments with rapid chitin particle turnover. This insight provides an environmentally relevant example of how cell morphology can impact bacterial fitness.
Keywords: Vibrio cholerae; biofilm; cell shape; chitin; extracellular matrix.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Settling down on chitin.Nat Rev Microbiol. 2019 Sep;17(9):527. doi: 10.1038/s41579-019-0237-y. Nat Rev Microbiol. 2019. PMID: 31289382 No abstract available.
References
-
- Flemming H.-C., et al. , Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575 (2016). - PubMed
-
- Rudrappa T., Biedrzycki M. L., Bais H. P., Causes and consequences of plant-associated biofilms. FEMS Microbiol. Ecol. 64, 153–166 (2008). - PubMed
-
- Ciofu O., Hansen C. R., Høiby N., Respiratory bacterial infections in cystic fibrosis. Curr. Opin. Pulm. Med. 19, 251–258 (2013). - PubMed
-
- Wolcott R., Disrupting the biofilm matrix improves wound healing outcomes. J. Wound Care 24, 366–371 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

