Circuit asymmetries underlie functional lateralization in the mouse auditory cortex
- PMID: 31239458
- PMCID: PMC6592910
- DOI: 10.1038/s41467-019-10690-3
Circuit asymmetries underlie functional lateralization in the mouse auditory cortex
Abstract
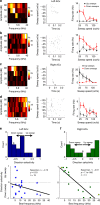
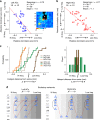
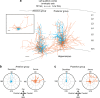
The left hemisphere's dominance in processing social communication has been known for over a century, but the mechanisms underlying this lateralized cortical function are poorly understood. Here, we compare the structure, function, and development of each auditory cortex (ACx) in the mouse to look for specializations that may underlie lateralization. Using Fos brain volume imaging, we found greater activation in the left ACx in response to vocalizations, while the right ACx responded more to frequency sweeps. In vivo recordings identified hemispheric differences in spectrotemporal selectivity, reinforcing their functional differences. We then compared the synaptic connectivity within each hemisphere and discovered lateralized circuit-motifs that are hearing experience-dependent. Our results suggest a specialist role for the left ACx, focused on facilitating the detection of specific vocalization features, while the right ACx is a generalist with the ability to integrate spectrotemporal features more broadly.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Differences in temporal processing speeds between the right and left auditory cortex reflect the strength of recurrent synaptic connectivity.PLoS Biol. 2022 Oct 21;20(10):e3001803. doi: 10.1371/journal.pbio.3001803. eCollection 2022 Oct. PLoS Biol. 2022. PMID: 36269764 Free PMC article.
-
Bilateral widefield calcium imaging reveals circuit asymmetries and lateralized functional activation of the mouse auditory cortex.Proc Natl Acad Sci U S A. 2023 Jul 25;120(30):e2219340120. doi: 10.1073/pnas.2219340120. Epub 2023 Jul 17. Proc Natl Acad Sci U S A. 2023. PMID: 37459544 Free PMC article.
-
Asynchronous development of the mouse auditory cortex is driven by hemispheric identity and sex.Nat Commun. 2025 Apr 17;16(1):3654. doi: 10.1038/s41467-025-58891-3. Nat Commun. 2025. PMID: 40246875 Free PMC article.
-
Common principles in the lateralization of auditory cortex structure and function for vocal communication in primates and rodents.Eur J Neurosci. 2022 Feb;55(3):827-845. doi: 10.1111/ejn.15590. Epub 2022 Jan 23. Eur J Neurosci. 2022. PMID: 34984748 Review.
-
Hemispheric asymmetries in cerebral cortical networks.Trends Neurosci. 2003 Aug;26(8):429-35. doi: 10.1016/S0166-2236(03)00198-X. Trends Neurosci. 2003. PMID: 12900174 Review.
Cited by
-
Development of Auditory Cortex Circuits.J Assoc Res Otolaryngol. 2021 Jun;22(3):237-259. doi: 10.1007/s10162-021-00794-3. Epub 2021 Apr 28. J Assoc Res Otolaryngol. 2021. PMID: 33909161 Free PMC article. Review.
-
The Temporal Association Cortex Plays a Key Role in Auditory-Driven Maternal Plasticity.Neuron. 2020 Aug 5;107(3):566-579.e7. doi: 10.1016/j.neuron.2020.05.004. Epub 2020 May 29. Neuron. 2020. PMID: 32473095 Free PMC article.
-
Sex, strain, and lateral differences in brain cytoarchitecture across a large mouse population.Elife. 2023 May 5;12:e82376. doi: 10.7554/eLife.82376. Elife. 2023. PMID: 37144870 Free PMC article.
-
Developmental encoding of natural sounds in the mouse auditory cortex.Cereb Cortex. 2024 Nov 5;34(11):bhae438. doi: 10.1093/cercor/bhae438. Cereb Cortex. 2024. PMID: 39503245 Free PMC article.
-
Differences in temporal processing speeds between the right and left auditory cortex reflect the strength of recurrent synaptic connectivity.PLoS Biol. 2022 Oct 21;20(10):e3001803. doi: 10.1371/journal.pbio.3001803. eCollection 2022 Oct. PLoS Biol. 2022. PMID: 36269764 Free PMC article.
References
-
- Broca P. Remarques sur le sie’ge de la faculte’ du language articule’, suivies d’une observation d’aphe’mie (perte de la parole) Bull. Soc. Anat. 1861;6:330–357.
-
- Ocklenburg, S., Ströckens, F. & Güntürkün, O. Lateralisation of conspecific vocalisation in non-human vertebrates. Laterality18, 1–31 (2011). - PubMed
Publication types
MeSH terms
Grants and funding
- G12 MD007603/MD/NIMHD NIH HHS/United States
- 5G12MD007603/U.S. Department of Health & Human Services | NIH | National Institute on Minority Health and Health Disparities (NIMHD)/International
- IOS-1652774/National Science Foundation (NSF)/International
- 2014-12-80/Whitehall Foundation (Whitehall Foundation, Inc.)/International
LinkOut - more resources
Full Text Sources
Miscellaneous

