Inhibition of the Receptor for Advanced Glycation End-Products in Acute Respiratory Distress Syndrome: A Randomised Laboratory Trial in Piglets
- PMID: 31239497
- PMCID: PMC6592897
- DOI: 10.1038/s41598-019-45798-5
Inhibition of the Receptor for Advanced Glycation End-Products in Acute Respiratory Distress Syndrome: A Randomised Laboratory Trial in Piglets
Abstract
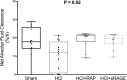
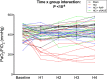
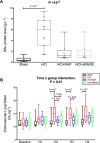
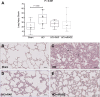
The receptor for advanced glycation end-products (RAGE) modulates the pathogenesis of acute respiratory distress syndrome (ARDS). RAGE inhibition attenuated lung injury and restored alveolar fluid clearance (AFC) in a mouse model of ARDS. However, clinical translation will require assessment of this strategy in larger animals. Forty-eight anaesthetised Landrace piglets were randomised into a control group and three treatment groups. Animals allocated to treatment groups underwent orotracheal instillation of hydrochloric acid (i) alone; (ii) in combination with intravenous administration of a RAGE antagonist peptide (RAP), or (iii) recombinant soluble (s)RAGE. The primary outcome was net AFC at 4 h. Arterial oxygenation was assessed hourly and alveolar-capillary permeability, alveolar inflammation and lung histology were assessed at 4 h. Treatment with either RAP or sRAGE improved net AFC (median [interquartile range], 21.2 [18.8-21.7] and 19.5 [17.1-21.5] %/h, respectively, versus 12.6 [3.2-18.8] %/h in injured, untreated controls), oxygenation and decreased alveolar inflammation and histological evidence of tissue injury after ARDS. These findings suggest that RAGE inhibition restored AFC and attenuated lung injury in a piglet model of acid-induced ARDS.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Fan E, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. - DOI - PubMed
-
- Force ARDSDefinitionTask, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

