Inhibition of tumor metastasis by targeted daunorubicin and dioscin codelivery liposomes modified with PFV for the treatment of non-small-cell lung cancer
- PMID: 31239668
- PMCID: PMC6551515
- DOI: 10.2147/IJN.S194304
Inhibition of tumor metastasis by targeted daunorubicin and dioscin codelivery liposomes modified with PFV for the treatment of non-small-cell lung cancer
Abstract
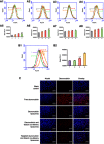
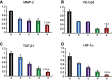
Background: Chemotherapy for non-small-cell lung cancer (NSCLC) still leads to unsatisfactory clinical prognosis because of poor active targeting and tumor metastasis. Purpose: The objective of this study was to construct a kind of PFV peptide modified targeted daunorubicin and dioscin codelivery liposomes, which could enhance tumor targeting and inhibit tumor cell metastasis. Methods and results: Targeted daunorubicin and dioscin codelivery liposomes were prepared by film dispersion and the ammonium sulfate gradient method. With the ideal physicochemical properties, targeted daunorubicin and dioscin codelivery liposomes exhibited enhanced cellular uptake and showed strong cytotoxicity to tumor cells. The encapsulation of dioscin increased the inhibitory effects of daunorubicin on A549 cells, vasculogenic mimicry (VM) channels and tumor metastasis. The enhanced antimetastatic mechanism of the targeted liposomes was attributed to the downregulation of matrix metalloproteinase-2 (MMP-2), vascular endothelial cadherin (VE-Cad), transforming growth factor-β1 (TGF-β1) and hypoxia inducible factor-1α (HIF-1α). Meanwhile, the targeted daunorubicin and dioscin codelivery liposomes exhibited significant antitumor effects in tumor-bearing mice. H&E staining, immunohistochemistry with Ki-67 and TUNEL assay also showed the promoted antitumor activity of the targeted liposomes. Conclusion: Targeted daunorubicin and dioscin codelivery liposomes may provide an effective strategy for the treatment of NSCLC.
Keywords: cell penetrating peptides; daunorubicin; dioscin; liposomes; non-small-cell lung cancer; tumor metastasis.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

