A pooled analysis of transarterial radioembolization with yttrium-90 microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma
- PMID: 31239717
- PMCID: PMC6560193
- DOI: 10.2147/OTT.S202875
A pooled analysis of transarterial radioembolization with yttrium-90 microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma
Abstract
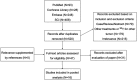
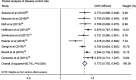
Purpose: The aim of this pooled analysis was to evaluate the clinical efficacy and safety of transarterial radioembolization (TARE) with yttrium-90 (90Y) microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma (ICC). Methods: We searched the Cochrane Library, Embase, PubMed, SCI with the English language from inception to October 2018. A pooled analysis was conducted using Stata software. Results: There were 16 eligible studies included in this pooled analysis. The pooled median overall survival (OS) from 12 studies was 14.3 (95% CI: 11.9-17.1) months. Based on Response Evaluation Criteria in Solid Tumors (RECIST), no complete response was reported, and the median of partial response, stable disease and progressive disease were 11.5% (range: 4.8-35.3%), 61.5% (range: 42.9-81.3%) and 22.7% (range: 12.5-52.4%) respectively. The pooled disease control rate (DCR) from nine studies was 77.2% (95% CI: 70.2-84.2%). According to the type of microspheres, subgroup analysis was performed, the median OS in the glass microspheres group was 14.0 (95% CI: 9.1-21.4) months, and 14.3 (95% CI: 11.5-17.8) months in the resin microspheres group. The DCR was 77.3% (95% CI: 63.5-91.1%) and 77.4% (95% CI: 66.8-87.9%) in the glass and resin microspheres groups respectively. Most of the side effects reported in the included studies were mild and did not require intervention. Conclusion: TARE with 90Y microspheres is safe and effective for patients with unresectable ICC with acceptable side effects. And it seems that the type of microsphere has no influence on therapeutic efficacy.
Keywords: intrahepatic cholangiocarcinoma; pooled analysis; transarterial radioembolization; yttrium-90 microspheres.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Medical