Current status of liquid biopsies for the detection and management of prostate cancer
- PMID: 31239778
- PMCID: PMC6559244
- DOI: 10.2147/CMAR.S170380
Current status of liquid biopsies for the detection and management of prostate cancer
Abstract
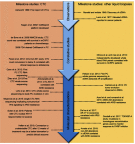
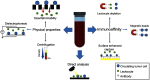
In recent years, new therapeutic options have become available for prostate cancer (PC) patients, generating an urgent need for better biomarkers to guide the choice of therapy and monitor treatment response. Liquid biopsies, including circulating tumor cells (CTCs), circulating nucleic acids, and exosomes, have been developed as minimally invasive assays allowing oncologists to monitor PC patients with real-time cellular or molecular information. While CTC counts remain the most extensively validated prognostic biomarker to monitor treatment response, recent advances demonstrate that CTC morphology and androgen receptor characterization can provide additional information to guide the choice of treatment. Characterization of cell-free DNA (cfDNA) is another rapidly emerging field with novel technologies capable of monitoring the evolution of treatment relevant alterations such as those in DNA damage repair genes for poly (ADP-ribose) polymerase (PARP) inhibition. In addition, several new liquid biopsy fields are emerging, including the characterization of heterogeneity, CTC RNA sequencing, the culture and xenografting of CTCs, and the characterization of extracellular vesicles (EVs) and circulating microRNAs. This review describes the clinical utilization of liquid biopsies in the management of PC patients and emerging liquid biopsy technologies with the potential to advance personalized cancer therapy.
Keywords: biomarker; circulating tumor DNA; circulating tumor cell; prostate cancer.
Conflict of interest statement
Dr. Amir Goldkorn reports personal fees from Acadia Woods (VC firm) outside the submitted work; In addition, Dr. Amir Goldkorn has a patent US8551425B2: Method for cancer detection, diagnosis, and prognosis licensed to Circulogix, Corestone. The authors report no other conflicts of interest in this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

