Redox Signaling in Sickle Cell Disease
- PMID: 31240269
- PMCID: PMC6592428
- DOI: 10.1016/j.cophys.2019.04.022
Redox Signaling in Sickle Cell Disease
Abstract
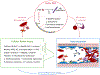
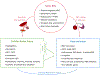
Sickle cell disease (SCD) is characterized by chronic hemolysis and repeated episodes of vascular occlusion leading to progressive organ injury. SCD is characterized by unbalanced, simultaneous pro-oxidant and anti-oxidant processes at the molecular, cellular and tissue levels, with the majority of reactions tipped in favor of pro-oxidant pathways. In this brief review we discuss new findings regarding how oxidized hemin, hemolysis, mitochondrial dysfunction and the innate immune system generate oxidative stress while hemopexin, haptoglobin, heme oxygenase-1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2) may provide protection in human and murine SCD. We will also describe recent clinical trials showing beneficial effects of antioxidant therapy in SCD.
Keywords: hemoglobin; hemolysis; inflammation; oxidative stress; sickle cell disease.
Conflict of interest statement
Declaration of Interests: None
Figures
References
-
- Camus SM, Gausseres B, Bonnin P, Loufrani L, Grimaud L, Charue D, De Moraes JA, Renard JM, Tedgui A, Boulanger CM, Tharaux PL, Blanc-Brude OP. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood. 2012; 120(25): 5050–8. doi: 10.1182/blood-2012-02-413138. PubMed PMID: 22976952. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
