Open source acoustofluidics
- PMID: 31240285
- PMCID: PMC6934416
- DOI: 10.1039/c9lc00340a
Open source acoustofluidics
Abstract
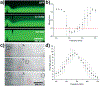
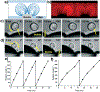
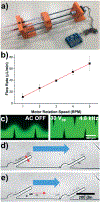
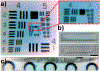
Over the past several decades, a litany of acoustofluidic devices have been developed which purport to have significant advantages over traditional benchtop analytical tools. These acoustofluidic devices are frequently labeled as "labs-on-chips"; however, many do an insufficient job of limiting their dependence on the lab. Often, acoustofluidic devices still require skilled operators and complex external equipment. In an effort to address these shortcomings, we developed a low-cost, expandable, and multifunctional system for controlling acoustofluidic devices in the audible to low ultrasonic frequency range (31 Hz to 65 kHz). The system was designed around the readily available Arduino prototyping platform because of its user-friendly coding environment and expansive network of open source material; these factors enabled us to create a system capable of generating high voltage oscillatory signals and controlling microscale flows in acoustofluidic devices. Utilizing the established open source system, we achieved a series of acoustofluidic applications involving the manipulation of fluids and biological objects in a portable fashion. In particular, we used our open source acoustofluidic devices to achieve active rotation of cells and microorganisms, and operation of an acoustofluidic mixing device which has previously shown potential for viscous sample preparation, in a portable fashion. Additionally, using low frequency flexural waves and our portable system, we achieved acoustofluidic separation of particles based on size. It is our hope that the open source platform presented here can pave the way for future acoustofluidic devices to be used at the point-of-care, as well as simplify the operation of these devices to enable resource limited users to leverage the benefits of acoustofluidics in their work.
Conflict of interest statement
Conflicts of interest
No conflicts to declare.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

