Autoimmune Epilepsy
- PMID: 31240596
- PMCID: PMC6694338
- DOI: 10.1007/s13311-019-00750-3
Autoimmune Epilepsy
Abstract
The field of autoimmune epilepsy has evolved substantially in the last few decades with discovery of several neural autoantibodies and improved mechanistic understanding of these immune-mediated syndromes. A considerable proportion of patients with epilepsy of unknown etiology have been demonstrated to have an autoimmune cause. The majority of the patients with autoimmune epilepsy usually present with new-onset refractory seizures along with subacute progressive cognitive decline and behavioral or psychiatric dysfunction. Neural specific antibodies commonly associated with autoimmune epilepsy include leucine-rich glioma-inactivated protein 1 (LGI1), N-methyl-D-aspartate receptor (NMDA-R), and glutamic acid decarboxylase 65 (GAD65) IgG. Diagnosis of these cases depends on the identification of the clinical syndrome and ancillary studies including autoantibody evaluation. Predictive models (Antibody Prevalence in Epilepsy and Encephalopathy [APE2] and Response to Immunotherapy in Epilepsy and Encephalopathy [RITE2] scores) based on clinical features and initial neurological assessment may be utilized for selection of cases for autoimmune epilepsy evaluation and management. In this article, we will review the recent advances in autoimmune epilepsy and provide diagnostic and therapeutic algorithms for epilepsies with suspected autoimmune etiology.
Keywords: Autoimmune limbic encephalitis; Diagnosis; Epilepsy; Immunotherapy.
Figures

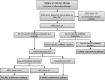
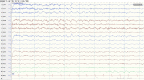
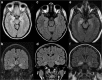
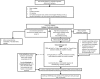
References
-
- Brodie MJ, et al. The 2017 ILAE classification of seizure types and the epilepsies: what do people with epilepsy and their caregivers need to know? Epileptic Disord. 2018;20(2):77–87. - PubMed
-
- Dubey D, Pittock SJ, McKeon A. Antibody Prevalence in Epilepsy and Encephalopathy score: Increased specificity and applicability. Epilepsia. 2019;60(2):367–369. - PubMed
-
- Dubey D, et al. Neurological Autoantibody Prevalence in Epilepsy of Unknown Etiology. JAMA Neurol. 2017;74(4):397–402. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

