Prevention of postsurgical lymphedema via immediate delivery of sustained-release 9-cis retinoic acid to the lymphedenectomy site
- PMID: 31240729
- PMCID: PMC6930358
- DOI: 10.1002/jso.25587
Prevention of postsurgical lymphedema via immediate delivery of sustained-release 9-cis retinoic acid to the lymphedenectomy site
Abstract
Background and objectives: Previously, we have shown that 9-cis retinoic acid (9-cis RA) stimulates lymphangiogenesis and limits postsurgical lymphedema in animal models when administered via daily intraperitoneal injections. In this study, we investigate whether a single-use depot 9-cis RA drug delivery system (DDS) implanted at the site of lymphatic injury can mitigate the development of lymphedema in a clinically relevant mouse limb model.
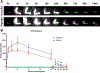
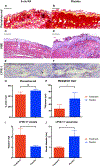
Methods: Hind limb lymphedema was induced via surgical lymphadenectomy and irradiation. Animals were divided into two treatment groups: (1) 9-cis RA DDS, (2) placebo DDS. Outcomes measured included paw thickness, lymphatic clearance and density, epidermal thickness, and collagen deposition.
Results: Compared with control animals, 9-cis RA-treated animals had significantly less paw swelling from postoperative week 3 (P = .04) until the final timepoint at week 6 (P = .0007). Moreover, 9-cis RA-treated animals had significantly faster lymphatic clearance (P < .05), increased lymphatic density (P = .04), reduced lymphatic vessel size (P = .02), reduced epidermal hyperplasia (P = .04), and reduced collagen staining (P = .10).
Conclusions: Animals receiving 9-cis RA sustained-release implants at the time of surgery had improved lymphatic function and structure, indicating reduced lymphedema progression. Thus, we demonstrate that 9-cis RA contained within a single-use depot DDS has favorable properties in limiting pathologic responses to lymphatic injury and may be an effective strategy against secondary lymphedema.
Keywords: 9-cis retinoic acid; depot; drug delivery system; drug pellet; lymphangiogenesis; lymphedema; postsurgical lymphedema; retinoic acid; secondary lymphedema; sustained release.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Disclosures
The authors have no conflicts of interest to disclose in relation to the contents of this manuscript.
Figures




References
-
- Rockson SG, Rivera KK (2008) Estimating the population burden of lymphedema. Ann N Y Acad Sci 1131: 147–154. - PubMed
-
- Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, et al. (2009) Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol 27: 2007–2014. - PubMed
-
- Dellon AL, Hoopes JE (1977) The Charles procedure for primary lymphedema. Long-term clinical results. Plast Reconstr Surg 60: 589–595. - PubMed
-
- Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, et al. (2002) Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 16: 1985–1987. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

