Temporal induction of intestinal epithelial hypoxia-inducible factor-2α is sufficient to drive colitis
- PMID: 31241981
- PMCID: PMC6734372
- DOI: 10.1152/ajpgi.00081.2019
Temporal induction of intestinal epithelial hypoxia-inducible factor-2α is sufficient to drive colitis
Abstract
Hypoxia is a notable feature of inflammatory bowel disease and chronic induction of hypoxia-inducible factor (HIF)-1α and HIF-2α (endothelial PAS domain protein 1, EPAS1) play important, but opposing, roles in its pathogenesis. While activation of HIF-1α decreases intestinal inflammation and is beneficial in colitis, activation of HIF-2α exacerbates colitis and increases colon carcinogenesis in animal models, primarily due to the role of epithelial HIF-2α in mounting a potent inflammatory response. Previous work from our laboratory showed that mice overexpressing intestinal epithelial HIF-2α led to massive intestinal inflammation and decreased survival. As oxygen homeostasis and HIFs are critical in embryonic development, it is not clear whether the observed intestinal inflammatory response was secondary to developmental defects. To address this question, the present study used a mouse model to temporally modulate expression of intestinal epithelial HIF-2α to assess its role in mediating inflammatory response. Remarkably, activation of HIF-2α in intestinal epithelial cells in adult mice increased expression of proinflammatory mediators; however, no decrease in survival was observed. Furthermore, in an acute model of colitis, activation of HIF-2α was sufficient to exacerbate colitis. These data confirm our previous finding that epithelial HIF-2α mediates inflammatory response and demonstrates that activation of HIF-2α is sufficient to exacerbate colitis.NEW & NOTEWORTHY Inflammatory bowel disease (IBD) is a chronic relapsing inflammatory disease of the intestinal tract. Hypoxia and activation of its downstream transcription factors hypoxia-inducible factor (HIF)-1α and HIF-2α are notable features of IBD. HIF-1α has well-characterized protective roles in IBD; however, the role of HIF-2α has been less studied. Using novel HIF-2α mouse models, we show that activation of HIF-2α in intestinal epithelial cells is sufficient to exacerbate colitis.
Keywords: colitis; hypoxia; hypoxia-inducible factor-2α.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
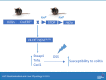


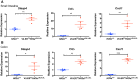
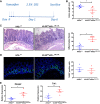

References
-
- Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40: 66–77, 2014. doi:10.1016/j.immuni.2013.11.020. - DOI - PMC - PubMed
-
- Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Hao G, Yousuf Q, Joyce A, Pedrosa I, Geiger H, Zhang H, Chang J, Gardner KH, Bruick RK, Reeves C, Hwang TH, Courtney K, Frenkel E, Sun X, Zojwalla N, Wong T, Rizzi JP, Wallace EM, Josey JA, Xie Y, Xie XJ, Kapur P, McKay RM, Brugarolas J. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539: 112–117, 2016. doi:10.1038/nature19796. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

