Interdependence of hypoxia and β-adrenergic receptor signaling in pulmonary arterial hypertension
- PMID: 31242023
- PMCID: PMC6766716
- DOI: 10.1152/ajplung.00015.2019
Interdependence of hypoxia and β-adrenergic receptor signaling in pulmonary arterial hypertension
Abstract
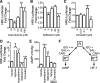
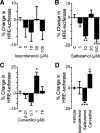
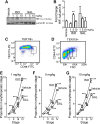
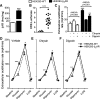
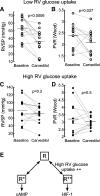
The β-adrenergic receptor (βAR) exists in an equilibrium of inactive and active conformational states, which shifts in response to different ligands and results in downstream signaling. In addition to cAMP, βAR signals to hypoxia-inducible factor 1 (HIF-1). We hypothesized that a βAR-active conformation (R**) that leads to HIF-1 is separable from the cAMP-activating conformation (R*) and that pulmonary arterial hypertension (PAH) patients with HIF-biased conformations would not respond to a cAMP agonist. We compared two cAMP agonists, isoproterenol and salbutamol, in vitro. Isoproterenol increased cAMP and HIF-1 activity, while salbutamol increased cAMP and reduced HIF-1. Hypoxia blunted agonist-stimulated cAMP, consistent with receptor equilibrium shifting toward HIF-activating conformations. Similarly, isoproterenol increased HIF-1 and erythropoiesis in mice, while salbutamol decreased erythropoiesis. βAR overexpression in cells increased glycolysis, which was blunted by HIF-1 inhibitors, suggesting increased βAR leads to increased hypoxia-metabolic effects. Because PAH is also characterized by HIF-related glycolytic shift, we dichotomized PAH patients in the Pulmonary Arterial Hypertension Treatment with Carvedilol for Heart Failure trial (NCT01586156) based on right ventricular (RV) glucose uptake to evaluate βAR ligands. Patients with high glucose uptake had more severe disease than those with low uptake. cAMP increased in response to isoproterenol in mononuclear cells from low-uptake patients but not in high-uptake patients' cells. When patients were treated with carvedilol for 1 wk, the low-uptake group decreased RV systolic pressures and pulmonary vascular resistance, but high-uptake patients had no physiologic responses. The findings expand the paradigm of βAR activation and uncover a novel PAH subtype that might benefit from β-blockers.
Keywords: hypoxia; metabolism; pulmonary hypertension; β-adrenergic receptor.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Fasting 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year.Ann Am Thorac Soc. 2013 Feb;10(1):1-9. doi: 10.1513/AnnalsATS.201206-029OC. Ann Am Thorac Soc. 2013. PMID: 23509326 Free PMC article.
-
β3 adrenergic agonism: A novel pathway which improves right ventricular-pulmonary arterial hemodynamics in pulmonary arterial hypertension.Physiol Rep. 2023 Jan;11(1):e15549. doi: 10.14814/phy2.15549. Physiol Rep. 2023. PMID: 36597221 Free PMC article.
-
The selective PGI2 receptor agonist selexipag ameliorates Sugen 5416/hypoxia-induced pulmonary arterial hypertension in rats.PLoS One. 2020 Oct 15;15(10):e0240692. doi: 10.1371/journal.pone.0240692. eCollection 2020. PLoS One. 2020. PMID: 33057388 Free PMC article.
-
The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure.Circ Res. 2014 Jun 20;115(1):176-88. doi: 10.1161/CIRCRESAHA.113.301129. Circ Res. 2014. PMID: 24951766 Free PMC article. Review.
-
Beta-blockers in pulmonary arterial hypertension: Time for a second thought?Vascul Pharmacol. 2022 Jun;144:106974. doi: 10.1016/j.vph.2022.106974. Epub 2022 Mar 4. Vascul Pharmacol. 2022. PMID: 35248781 Review.
Cited by
-
Taking it to heart: dissecting cardiopulmonary interactions in diseases of the lung and the cardiovascular system.Am J Physiol Lung Cell Mol Physiol. 2020 Sep 1;319(3):L547-L549. doi: 10.1152/ajplung.00373.2020. Epub 2020 Aug 12. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32783622 Free PMC article. No abstract available.
-
Novel Therapeutic Targets for the Treatment of Right Ventricular Remodeling: Insights from the Pulmonary Artery Banding Model.Int J Environ Res Public Health. 2021 Aug 5;18(16):8297. doi: 10.3390/ijerph18168297. Int J Environ Res Public Health. 2021. PMID: 34444046 Free PMC article. Review.
-
Hypoxia responses in arginase 2 deficient mice enhance cardiovascular health.bioRxiv [Preprint]. 2025 Mar 10:2025.02.20.639297. doi: 10.1101/2025.02.20.639297. bioRxiv. 2025. PMID: 40060685 Free PMC article. Preprint.
-
Hypoxia Sensing of β-Adrenergic Receptor Is Regulated by Endosomal PI3Kγ.Circ Res. 2023 Mar 17;132(6):690-703. doi: 10.1161/CIRCRESAHA.122.321735. Epub 2023 Feb 13. Circ Res. 2023. PMID: 36779349 Free PMC article.
-
Metabolomics Meets Clinics: A Multivariate Analysis of Plasma and Urine Metabolic Signatures in Pulmonary Arterial Hypertension.J Proteome Res. 2024 Aug 2;23(8):2795-2804. doi: 10.1021/acs.jproteome.3c00255. Epub 2023 Oct 12. J Proteome Res. 2024. PMID: 37827514 Free PMC article.
References
-
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G. β-Arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100: 11406–11411, 2003. doi:10.1073/pnas.1936664100. - DOI - PMC - PubMed
-
- Bouvier M, Collins S, O’Dowd BF, Campbell PT, de Blasi A, Kobilka BK, MacGregor C, Irons GP, Caron MG, Lefkowitz RJ. Two distinct pathways for cAMP-mediated down-regulation of the beta 2-adrenergic receptor. Phosphorylation of the receptor and regulation of its mRNA level. J Biol Chem 264: 16786–16792, 1989. - PubMed
-
- Bristow MR, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein JW, Anderson FL, Murray J, Mestroni L, Karwande SV. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 89: 803–815, 1992. doi:10.1172/JCI115659. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

