Synbiotics suppress colitis-induced tumorigenesis in a colon-specific cancer mouse model
- PMID: 31242213
- PMCID: PMC6594584
- DOI: 10.1371/journal.pone.0216393
Synbiotics suppress colitis-induced tumorigenesis in a colon-specific cancer mouse model
Abstract
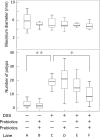
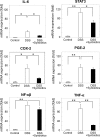
Although synbiotics may be effective in maintaining remission of inflammatory bowel disease, their anticarcinogenic effects are still debated. To address this issue, we evaluated the effects of synbiotics, probiotics, and prebiotics on tumorigenesis using a CDX2P-Cre; Apc+/flox mouse model harboring a colon-specific Apc knock out, which develops adenoma and adenocarcinoma of the colon. Dextran sodium sulfate (DSS)-administration promoted colonic tumor development in CDX2P-Cre; Apc+/flox mice, and these tumors were associated with loss of Apc heterozygosity, as confirmed by observation of well-differentiated adenocarcinomas with β-catenin accumulation in tumor cell cytoplasm. Synbiotics-treatment suppressed dextran sodium sulfate-induced colitis in CDX2P-Cre; Apc+/flox mice, thereby reducing mortality, and inhibited tumorigenesis accelerated by DSS-administration. Conversely, neither probiotics nor prebiotics had any effect on inflammation and tumorigenesis. Lactobacillus casei and Bifidobacterium breve were detected in the fecal microbiota of probiotics-treated mice. Synbiotics-treatment suppressed DSS-induced expression of IL-6, STAT-3, COX-2, and TNF-α gene transcripts in normal colonic epithelium, indicating the possibility of suppressing tumor development. Importantly, these genes may be potential therapeutic targets in inflammation-associated colon cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res. 2010;3: 1314–1333. - PubMed
-
- Rosenstock E, Farmer RG, Petras R, Sivak MV Jr, Rankin GB, Sullivan BH. Surveillance for colonic carcinoma in ulcerative colitis. Gastroenterology. 1985;89: 1342–1346. - PubMed
-
- Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: Analysis of changes in disease activity over years. Gastroenterology. 1994;107: 3–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

