Physical and Molecular Landscapes of Mouse Glioma Extracellular Vesicles Define Heterogeneity
- PMID: 31242427
- PMCID: PMC6604862
- DOI: 10.1016/j.celrep.2019.05.089
Physical and Molecular Landscapes of Mouse Glioma Extracellular Vesicles Define Heterogeneity
Abstract
Cancer extracellular vesicles (EVs) are highly heterogeneous, which impedes our understanding of their function as intercellular communication agents and biomarkers. To deconstruct this heterogeneity, we analyzed extracellular RNAs (exRNAs) and extracellular proteins (exPTNs) from size fractionation of large, medium, and small EVs and ribonucleoprotein complexes (RNPs) from mouse glioblastoma cells by RNA sequencing and quantitative proteomics. mRNA from medium-sized EVs most closely reflects the cellular transcriptome, whereas small EV exRNA is enriched in small non-coding RNAs and RNPs contain precisely processed tRNA fragments. The exPTN composition of EVs and RNPs reveals that they are closely related by vesicle type, independent of their cellular origin, and single EV analysis reveals that small EVs are less heterogeneous in their protein content than larger ones. We provide a foundation for better understanding of segregation of macromolecules in glioma EVs through a catalog of diverse exRNAs and exPTNs.
Keywords: exosomes; extracellular RNA; extracellular vesicles; glioblastoma; mouse model; proteome.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures
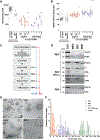

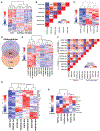




References
-
- Balatti V, Pekarsky Y, and Croce CM (2017). Role of the tRNA-Derived Small RNAs in Cancer: New Potential Biomarkers and Target for Therapy. Adv. Cancer Res. 135, 173–187. - PubMed
-
- Beausoleil SA, Villén J, Gerber SA, Rush J, and Gygi SP (2006). A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol 24, 1285–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

