Dorsomedial prefrontal cortex neurons encode nicotine-cue associations
- PMID: 31242502
- PMCID: PMC6898138
- DOI: 10.1038/s41386-019-0449-x
Dorsomedial prefrontal cortex neurons encode nicotine-cue associations
Abstract
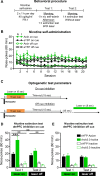
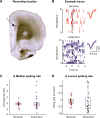
The role of medial prefrontal cortex (mPFC) in regulating nicotine taking and seeking remains largely unexplored. In this study we took advantage of the high time-resolution of optogenetic intervention by decreasing (Arch3.0) or increasing (ChR2) the activity of neurons in the dorsal and ventral mPFC during 5-s nicotine cue presentations in order to evaluate their contribution to cued nicotine seeking and taking. Wistar rats were trained to self-administer intravenous nicotine in 1 h self-administration sessions twice a day for a minimum of 10 days. Subsequently, dmPFC or vmPFC neuronal activity was modulated during or following presentation of the 5-s nicotine cue, both under extinction and self-administration conditions. We also used in vivo electrophysiology to record the activity of dmPFC neurons during nicotine self-administration and extinction tests. We show that optogenetic inhibition of dmPFC neurons during, but not following, response-contingent presentations of the nicotine cue increased nicotine seeking. We found no effect on nicotine self-administration or on food seeking in an extinction test. We also show that this effect is specific to dmPFC, because optogenetic inhibition of vmPFC had no effect on nicotine seeking and taking. In vivo recordings revealed that dmPFC network neuronal activity was modulated more strongly following nicotine cue presentation in extinction, compared to following nicotine self-administration. Our results strongly suggest that a population of neurons within the dmPFC is involved in encoding the incentive value of nicotine-associated cues.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
Response-contingent optogenetics to discover the mechanisms of nicotine-cue associations.Neuropsychopharmacology. 2019 Nov;44(12):1995-1996. doi: 10.1038/s41386-019-0469-6. Epub 2019 Aug 5. Neuropsychopharmacology. 2019. PMID: 31383936 Free PMC article. No abstract available.
References
-
- World Health Organization. WHO report on the global tobacco epidemic: raising taxes on tobacco. World Health Organization. 2015. p. 52–53.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

