Design, Synthesis and Biological Evaluation of 7-Chloro-9 H-pyrimido[4,5- b]indole-based Glycogen Synthase Kinase-3β Inhibitors
- PMID: 31242571
- PMCID: PMC6630214
- DOI: 10.3390/molecules24122331
Design, Synthesis and Biological Evaluation of 7-Chloro-9 H-pyrimido[4,5- b]indole-based Glycogen Synthase Kinase-3β Inhibitors
Abstract
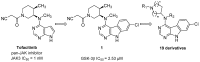
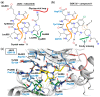
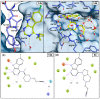
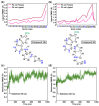
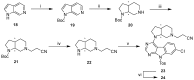
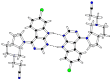
Glycogen synthase kinase-3β (GSK-3β) represents a relevant drug target for the treatment of neurodegenerative pathologies including Alzheimer's disease. We herein report on the optimization of a novel class of GSK-3β inhibitors based on the tofacitinib-derived screen hit 3-((3R,4R)-3-((7-chloro-9H-pyrimido[4,5-b]indol-4-yl)(methyl)amino)-4-methylpiperidin-1-yl)-3-oxopropanenitrile (1). We synthesized a series of 19 novel 7-chloro-9H-pyrimido[4,5-b]indole-based derivatives and studied their structure-activity relationships with focus on the cyanoacetyl piperidine moiety. We unveiled the crucial role of the nitrile group and its importance for the activity of this compound series. A successful rigidization approach afforded 3-(3aRS,7aSR)-(1-(7-chloro-9H-pyrimido[4,5-b]indol-4-yl)octahydro-6H-pyrrolo[2,3-c]pyridin-6-yl)-propanenitrile (24), which displayed an IC50 value of 130 nM on GSK-3β and was further characterized by its metabolic stability. Finally, we disclosed the putative binding modes of the most potent inhibitors within the ATP binding site of GSK-3β by 1 µs molecular dynamics simulations.
Keywords: 7-chloro-9H-pyrimido[4,5-b]indole; Glycogen synthase kinase-3β; kinase inhibitor; protein kinase; tofacitinib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
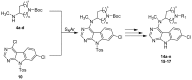
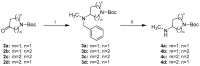
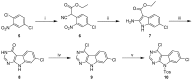
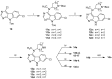
References
-
- Manning G., Genomic Overview of Protein Kinases. WormBook The C. Elegans Research Community, WormBook. [(accessed on 13 December 2005)]; doi: 10.1895/wormbook.1.60.1. Available online: http://www.wormbook.org. - DOI - PMC - PubMed
-
- Wang Y., Roach P.J. Inactivation of rabbit muscle glycogen synthase by glycogen synthase kinase-3. Dominant role of the phosphorylation of Ser-640 (site-3a) J. Biol. Chem. 1993;268:23876–23880. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

