A Free-Operant Reward-Tracking Paradigm to Study Neural Mechanisms and Neurochemical Modulation of Adaptive Behavior in Rats
- PMID: 31242610
- PMCID: PMC6627494
- DOI: 10.3390/ijms20123098
A Free-Operant Reward-Tracking Paradigm to Study Neural Mechanisms and Neurochemical Modulation of Adaptive Behavior in Rats
Abstract
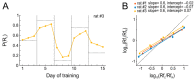
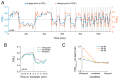
The ability to respond flexibly to changing environmental circumstances is a hallmark of goal-directed behavior, and compromised flexibility is associated with a wide range of psychiatric conditions in humans, such as addiction and stress-related disorders. To identify neural circuits and transmitter systems implicated in the provision of cognitive flexibility, suitable animal paradigms are needed. Ideally, such models should be easy to implement, allow for rapid task acquisition, provide multiple behavioral readouts, and permit combination with physiological and pharmacological testing and manipulation. Here, we describe a paradigm meeting these requirements and employ it to investigate the neural substrates and neurochemical modulation of adaptive behavior. Water-restricted rats learned to emit operant responses for positive reinforcement (water reward) within minutes in a free-operant conditioning environment. Without further training, animals were able to track changes in the reward schedule. Given prior evidence that the medial prefrontal cortex (mPFC) and the dopaminergic system are required for flexible behavior, we aimed to assess both in more detail. Silencing of mPFC compromised flexible behavior when avoidance of punishment was required. Systemic injections of the D2-receptor agonist quinpirole and the D2-receptor antagonist eticlopride had complex, differential impacts on reward seeking and adaptive behavior.
Keywords: dopamine receptors; matching law; muscimol; operant conditioning; punishment; reversal learning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Stad F.E., Vogelaar B., Bakker M., Resing W.C.M., Wiedl K.H. The role of cognitive flexibility in young children’s potential for learning under dynamic testing conditions. Eur. J. Psychol. Educ. 2018;34:123–146. doi: 10.1007/s10212-018-0379-8. - DOI
-
- Kercood S., Lineweaver T.T., Frank C.C., Fromm E.D. Cognitive Flexibility and Its Relationship to Academic Achievement and Career Choice of College Students With and Without Attention Deficit Hyperactivity Disorder. J. Postsecond. Educ. Disabil. 2017;30:329.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

