Eye Opener in Stroke
- PMID: 31242827
- PMCID: PMC6650274
- DOI: 10.1161/STROKEAHA.119.025249
Eye Opener in Stroke
Erratum in
-
Correction to: Eye Opener in Stroke: Mitochondrial Dysfunction and Stem Cell Repair in Retinal Ischemia.Stroke. 2022 Oct;53(10):e462. doi: 10.1161/STR.0000000000000416. Epub 2022 Sep 26. Stroke. 2022. PMID: 36154134 Free PMC article. No abstract available.
Abstract
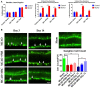
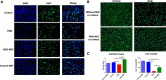
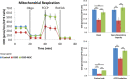
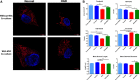
Background and Purpose- Retinal ischemia is a major cause of visual impairment in stroke patients, but our incomplete understanding of its pathology may contribute to a lack of effective treatment. Here, we investigated the role of mitochondrial dysfunction in retinal ischemia and probed the potential of mesenchymal stem cells (MSCs) in mitochondrial repair under such pathological condition. Methods- In vivo, rats were subjected to middle cerebral artery occlusion then randomly treated with intravenous MSCs or vehicle. Laser Doppler was used to evaluate the blood flow in the brain and the eye, while immunohistochemical staining assessed cellular degeneration at days 3 and 14 poststroke. In vitro, retinal pigmented epithelium cells were exposed to either oxygen-glucose deprivation or oxygen-glucose deprivation and coculture with MSCs, and subsequently, cell death and mitochondrial function were examined immunocytochemically and with Seahorse analyzer, respectively. Results- Middle cerebral artery occlusion significantly reduced blood flow in the brain and the eye accompanied by mitochondrial dysfunction and ganglion cell death at days 3 and 14 poststroke. Intravenous MSCs elicited mitochondrial repair and improved ganglion cell survival at day 14 poststroke. Oxygen-glucose deprivation similarly induced mitochondrial dysfunction and cell death in retinal pigmented epithelium cells; coculture with MSCs restored mitochondrial respiration, mitochondrial network morphology, and mitochondrial dynamics, which likely attenuated oxygen-glucose deprivation-mediated retinal pigmented epithelium cell death. Conclusions- Retinal ischemia is closely associated with mitochondrial dysfunction, which can be remedied by stem cell-mediated mitochondrial repair.
Keywords: cell survival; endothelial cells; glucose; mitochondrial dynamics; oxygen.
Figures


References
-
- Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. - PubMed
-
- Gumbinger C, Reuter B, Stock C, Sauer T, Wiethölter H, Bruder I, et al. AG Schlaganfall. Time to treatment with recombinant tissue plasminogen activator and outcome of stroke in clinical practice: retrospective analysis of hospital quality assurance data with comparison with results from randomised clinical trials. BMJ. 2014;348:g3429. doi: 10.1136/bmj.g3429. - PMC - PubMed
-
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. - PubMed
-
- Kaesmacher J, Kaesmacher M, Maegerlein C, Zimmer C, Gersing AS, Wunderlich S, et al. Hemorrhagic transformations after thrombectomy: risk factors and clinical relevance. Cerebrovasc Dis. 2017;43:294–304. doi: 10.1159/000460265. - PubMed
-
- Li Q, Gao X, Yao Z, Feng X, He H, Xue J, et al. Permeability surface of deep middle cerebral artery territory on computed tomographic perfusion predicts hemorrhagic transformation after stroke. Stroke. 2017;48:2412–2418. doi: 10.1161/STROKEAHA.117.017486. - PubMed

