Identification of Abundant and Evolutionarily Conserved Opioid Receptor Circular RNAs in the Nervous System Modulated by Morphine
- PMID: 31243060
- PMCID: PMC6666384
- DOI: 10.1124/mol.118.113977
Identification of Abundant and Evolutionarily Conserved Opioid Receptor Circular RNAs in the Nervous System Modulated by Morphine
Erratum in
-
Correction to "Identification of Abundant and Evolutionarily Conserved Opioid Receptor Circular RNAs in the Nervous System Modulated by Morphine".Mol Pharmacol. 2019 Nov;96(5):526. doi: 10.1124/mol.119.113977err. Mol Pharmacol. 2019. PMID: 31570532 Free PMC article. No abstract available.
Abstract
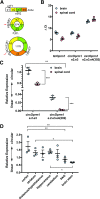
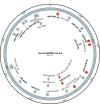
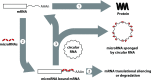
Circular RNAs (circRNAs) are a distinct category of single-stranded, covalently closed RNAs formed by backsplicing. The functions of circRNAs are incompletely known and are under active investigation. Here, we report that in addition to traditional linear mRNAs (linRNA), mouse, rat, and human opioid receptor genes generate exonic circRNA isoforms. Using standard molecular biologic methods, Oprm1 circRNAs (circOprm1) were detected in RNAs of rodent and human brains and spinal cords, as well as human neuroblastoma cells, suggesting evolutionary conservation. Sequencing confirmed backsplicing using canonical splice sites. Oprm1 circRNAs were sense-stranded circRNAs resistant to RNase R digestion. The relative abundance of Oprm1 circRNA to linRNA determined by quantitative reverse transcription polymerase chain reaction varied among mouse brain regions, with circRNA isoforms predominating in rostral structures and less abundant in brain stem. Chronic morphine exposure in mice increased brain circOprm1e2.3 and circOprm1.e2.e3.e4(302) levels by 1.5- to 1.6-fold relative to linRNA. Sequence analysis predicted numerous microRNA binding sites within Oprm1 circRNA sequences, suggesting a potential role in microRNA sequestration through sponging. In addition, we observed that other opioid receptor genes including δ, κ, and nociceptin receptor genes produced similar circRNAs. In conclusion, all members of the opioid receptor gene family express circRNAs, with Oprm1 circRNA levels exceeding those of linear forms in some regions. SIGNIFICANCE STATEMENT: The modulation of Oprm1 circular RNA (circRNA) expression by morphine, coupled with the high abundance and existence of potential miRNA binding sites with circRNA sequences suggests the potential role of Oprm1 circRNAs in chronic opioid effects such as tolerance.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





Similar articles
-
Alterations in Circular RNAs circOprm1 and circSerpini in the Striatum are Associated with Changes in Spatial Working Memory Performance after Morphine Dependence and Withdrawal in Rats.Neurochem Res. 2024 Nov 19;50(1):20. doi: 10.1007/s11064-024-04284-9. Neurochem Res. 2024. PMID: 39560876
-
Circular RNA expression profile in the spinal cord of morphine tolerated rats and screen of putative key circRNAs.Mol Brain. 2019 Sep 18;12(1):79. doi: 10.1186/s13041-019-0498-4. Mol Brain. 2019. PMID: 31533844 Free PMC article.
-
Merkel Cell Polyomavirus Encodes Circular RNAs (circRNAs) Enabling a Dynamic circRNA/microRNA/mRNA Regulatory Network.mBio. 2020 Dec 15;11(6):e03059-20. doi: 10.1128/mBio.03059-20. mBio. 2020. PMID: 33323517 Free PMC article.
-
Review on circular RNAs and new insights into their roles in cancer.Comput Struct Biotechnol J. 2021 Jan 22;19:910-928. doi: 10.1016/j.csbj.2021.01.018. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33598105 Free PMC article. Review.
-
Insights Into the Involvement of Circular RNAs in Autoimmune Diseases.Front Immunol. 2021 Feb 25;12:622316. doi: 10.3389/fimmu.2021.622316. eCollection 2021. Front Immunol. 2021. PMID: 33717126 Free PMC article. Review.
Cited by
-
Heroin Regulates Orbitofrontal Circular RNAs.Int J Mol Sci. 2022 Jan 27;23(3):1453. doi: 10.3390/ijms23031453. Int J Mol Sci. 2022. PMID: 35163373 Free PMC article.
-
Noncoding RNA therapeutics for substance use disorder.Adv Drug Alcohol Res. 2022;2:10807. doi: 10.3389/adar.2022.10807. Epub 2022 Dec 20. Adv Drug Alcohol Res. 2022. PMID: 36601439 Free PMC article.
-
Noncoding RNAs: Novel Targets for Opioid Tolerance.Curr Neuropharmacol. 2023;21(5):1202-1213. doi: 10.2174/1570159X21666221129122932. Curr Neuropharmacol. 2023. PMID: 36453497 Free PMC article. Review.
-
Alterations in Circular RNAs circOprm1 and circSerpini in the Striatum are Associated with Changes in Spatial Working Memory Performance after Morphine Dependence and Withdrawal in Rats.Neurochem Res. 2024 Nov 19;50(1):20. doi: 10.1007/s11064-024-04284-9. Neurochem Res. 2024. PMID: 39560876
-
Orbitofrontal intronic circular RNA from Nrxn3 mediates reward learning and motivation for reward.Prog Neurobiol. 2024 Jan;232:102546. doi: 10.1016/j.pneurobio.2023.102546. Epub 2023 Nov 29. Prog Neurobiol. 2024. PMID: 38036039 Free PMC article.
References
-
- Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. (2014) circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56:55–66. - PubMed
-
- Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. (1993) Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73:1019–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

