Analysis of Infection Loads in Mycoplasma genitalium Clinical Specimens by Use of a Commercial Diagnostic Test
- PMID: 31243085
- PMCID: PMC6711907
- DOI: 10.1128/JCM.00344-19
Analysis of Infection Loads in Mycoplasma genitalium Clinical Specimens by Use of a Commercial Diagnostic Test
Abstract
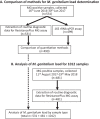
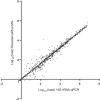
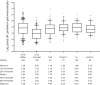
Mycoplasma genitalium is a common sexually transmitted infection with a propensity to acquire resistance to commonly used antimicrobial therapies. Bacterial load has been linked to patient symptoms and the success of treatment. In this study, we demonstrate methodology to estimate load from routine diagnostic assays using the ResistancePlus MG test (SpeeDx Pty Ltd., Australia). The method gave comparable quantitation to an M. genitalium-specific 16S rRNA quantitative PCR (qPCR; Spearman r = 0.94) for the samples analyzed (n = 499, including urine and swab types as detailed below) and was, therefore, employed to analyze typical load levels for samples in a diagnostic laboratory (total of 1,012 tests). When stratified by sample type, female urine (median, 826 genomes/ml) had the lowest load. This was significantly lower than median loads for all other sample types (male urine [6.91 × 103 genomes/ml], anal swabs [5.50 × 103], cervical swabs [8.15 × 103], endocervical swabs [3.97 × 103], and vaginal swabs [6.95 × 103]) (P < 0.0001). There were no significant differences in load estimates between the other sample types. Reproducibility of load estimates conducted on the same samples was high (r > 0.85). In conclusion, this methodology to provide load estimates for M. genitalium can be easily integrated into routine diagnostic laboratory workflow. Given the association between organism load, symptoms, and treatment success, load assessment has future diagnostic potential.
Keywords: Mycoplasma genitalium; diagnostic test; infection load; sample type; urine.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Read TRH, Fairley CK, Murray GL, Jensen JS, Danielewski J, Worthington K, Doyle M, Mokany E, Tan L, Chow EPF, Garland SM, Bradshaw CS. 2019. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis 68:554–560. doi:10.1093/cid/ciy477. - DOI - PMC - PubMed
-
- Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, Vodstrcil LA, Jensen JS, Hocking JS, Garland SM, Bradshaw CS. 2015. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 60:1228–1236. doi:10.1093/cid/ciu1162. - DOI - PubMed
-
- Walker J, Fairley CK, Bradshaw CS, Tabrizi SN, Twin J, Chen MY, Taylor N, Donovan B, Kaldor JM, McNamee K, Urban E, Walker S, Currie M, Birden H, Bowden FJ, Gunn J, Pirotta M, Gurrin L, Harindra V, Garland SM, Hocking JS. 2013. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin Infect Dis 56:1094–1100. doi:10.1093/cid/cis1210. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

