Transforming Growth Factor β Acts as a Regulatory Molecule for Lipogenic Pathways among Hepatitis C Virus Genotype-Specific Infections
- PMID: 31243135
- PMCID: PMC6714813
- DOI: 10.1128/JVI.00811-19
Transforming Growth Factor β Acts as a Regulatory Molecule for Lipogenic Pathways among Hepatitis C Virus Genotype-Specific Infections
Abstract
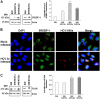
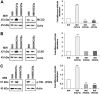
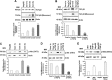
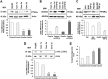
Hepatitis C virus (HCV) infection promotes metabolic disorders, and the severity of lipogenic disease depends upon the infecting virus genotype. Here, we have examined HCV genotype 1-, 2-, or 3-specific regulation of lipid metabolism, involving transforming growth factor β (TGF-β)-regulated phospho-Akt (p-Akt) and peroxisome proliferator-activated receptor alpha (PPARα) axes. Since HCV core protein is one of the key players in metabolic regulation, we also examined its contribution in lipid metabolic pathways. The expression of regulatory molecules, TGF-β1/2, phospho-Akt (Ser473), PPARα, sterol regulatory element-binding protein 1 (SREBP-1), fatty acid synthase (FASN), hormone-sensitive lipase (HSL), and acyl dehydrogenases was analyzed in virus-infected hepatocytes. Interestingly, HCV genotype 3a exhibited much higher activation of TGF-β and p-Akt, with a concurrent decrease in PPARα expression and fatty acid oxidation. A significant and similar decrease in HSL, unlike in HCV genotype 1a, was observed with both genotypes 2a and 3a. Similar observations were made from ectopic expression of the core genomic region from each genotype. The key role of TGF-β was further verified using specific small interfering RNA (siRNA). Together, our results highlight a significant difference in TGF-β-induced activity for the HCV genotype 2a- or 3a-induced lipogenic pathway, exhibiting higher triglyceride synthesis and a decreased lipolytic mechanism. These results may help in therapeutic modalities for early treatment of HCV genotype-associated lipid metabolic disorders.IMPORTANCE Hepatic steatosis is a frequent complication associated with chronic hepatitis C virus (HCV) infection and is a key prognostic indicator for progression to fibrosis and cirrhosis. Several mechanisms are proposed for the development of steatosis, especially with HCV genotype 3a. Our observations suggest that transforming growth factor β (TGF-β) and peroxisome proliferator-activated receptor alpha (PPARα)-associated mechanistic pathways in hepatocytes infected with HCV genotype 2a and 3a differ from those in cells infected with genotype 1a. The results suggest that a targeted therapeutic approach for enhanced PPARα and lipolysis may reduce HCV genotype-associated lipid metabolic disorder in liver disease.
Keywords: TGF-beta; core protein; hepatitis C virus; metabolic regulation.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Forkhead box transcription factor regulation and lipid accumulation by hepatitis C virus.J Virol. 2014 Apr;88(8):4195-203. doi: 10.1128/JVI.03327-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478438 Free PMC article.
-
Expression of genes involved in lipogenesis is not increased in patients with HCV genotype 3 in human liver.J Viral Hepat. 2011 Jan;18(1):53-60. doi: 10.1111/j.1365-2893.2010.01283.x. J Viral Hepat. 2011. PMID: 20196803
-
Hepatitis C virus genotype-3a core protein enhances sterol regulatory element-binding protein-1 activity through the phosphoinositide 3-kinase-Akt-2 pathway.J Gen Virol. 2010 Jun;91(Pt 6):1388-95. doi: 10.1099/vir.0.017418-0. Epub 2010 Feb 3. J Gen Virol. 2010. PMID: 20130133
-
Steatosis and insulin resistance in hepatitis C: a way out for the virus?World J Gastroenterol. 2009 Oct 28;15(40):5014-9. doi: 10.3748/wjg.15.5014. World J Gastroenterol. 2009. PMID: 19859993 Free PMC article. Review.
-
Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen.World J Gastroenterol. 2014 Jun 21;20(23):7089-103. doi: 10.3748/wjg.v20.i23.7089. World J Gastroenterol. 2014. PMID: 24966582 Free PMC article. Review.
Cited by
-
25-hydroxycholesterol: an integrator of antiviral ability and signaling.Front Immunol. 2023 Sep 13;14:1268104. doi: 10.3389/fimmu.2023.1268104. eCollection 2023. Front Immunol. 2023. PMID: 37781400 Free PMC article. Review.
-
Carvacrol, a Plant Metabolite Targeting Viral Protease (Mpro) and ACE2 in Host Cells Can Be a Possible Candidate for COVID-19.Front Plant Sci. 2021 Feb 16;11:601335. doi: 10.3389/fpls.2020.601335. eCollection 2020. Front Plant Sci. 2021. PMID: 33664752 Free PMC article. Review.
-
β-Caryophyllene, A Natural Dietary CB2 Receptor Selective Cannabinoid can be a Candidate to Target the Trinity of Infection, Immunity, and Inflammation in COVID-19.Front Pharmacol. 2021 May 14;12:590201. doi: 10.3389/fphar.2021.590201. eCollection 2021. Front Pharmacol. 2021. PMID: 34054510 Free PMC article.
-
Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19?Heliyon. 2020 Dec 11;7(1):e05703. doi: 10.1016/j.heliyon.2020.e05703. eCollection 2021 Jan. Heliyon. 2020. PMID: 33490659 Free PMC article. Review.
-
TGF-β isoforms inhibit hepatitis C virus propagation in transforming growth factor beta/SMAD protein signalling pathway dependent and independent manners.J Cell Mol Med. 2021 Apr;25(7):3498-3510. doi: 10.1111/jcmm.16432. Epub 2021 Mar 8. J Cell Mol Med. 2021. PMID: 33682288 Free PMC article.
References
-
- Castéra L, Hézode C, Roudot-Thoraval F, Lonjon I, Zafrani E-S, Pawlotsky J-M, Dhumeaux D. 2004. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut 53:420–424. doi:10.1136/gut.2002.009936. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

