The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal cancer
- PMID: 31243264
- PMCID: PMC6594974
- DOI: 10.1038/s41419-019-1687-x
The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal cancer
Abstract
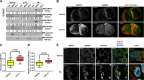
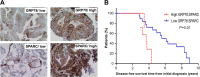
Therapy-refractory disease is one of the main contributors of treatment failure in cancer. In colorectal cancer (CRC), SPARC can function as a sensitizer to conventional chemotherapy by enhancing apoptosis by interfering with the activity of Bcl-2. Here, we examine a novel mechanism by which SPARC further potentiates apoptosis via its modulation of the unfolded protein response (UPR). Using mass spectrometry to identify SPARC-associated proteins, GRP78 was identified as a protein partner for SPARC in CRC. In vitro studies conducted to assess the signaling events resulting from this interaction, included induction of ER stress with tunicamycin, 5-fluorouracil (5-FU), and irinotecan (CPT-11). We found that the interaction between GRP78 and SPARC increased during exposure to 5-FU, CPT-11, and tunicamycin, resulting in an attenuation of GRP78's inhibition of apoptosis. In addition, we also show that SPARC can sensitize CRC cells to PERK/eIF2α and IRE1α/XBP-1 UPR signaling by interfering with ER stress following binding to GRP78, which leads to ER stress-associated cell death in CRC cells. In line with these findings, a lower expression of GRP78 relative to SPARC in CRC is associated with a lower IC50 for 5-FU in either sensitive or therapy-refractory CRC cells. Interestingly, this observation correlates with tissue microarray analysis of 143 human CRC, where low GRP78 to SPARC expression level was prognostic of higher survival rate (P = 0.01) in individuals with CRC. This study demonstrates that modulation of UPR signaling by SPARC promotes ER stress-associated death and potentiates apoptosis. This may be an effective strategy that can be combined with current treatment options to improve therapeutic efficacy in CRC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

