High-dimensional geometry of population responses in visual cortex
- PMID: 31243367
- PMCID: PMC6642054
- DOI: 10.1038/s41586-019-1346-5
High-dimensional geometry of population responses in visual cortex
Abstract
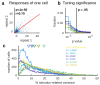
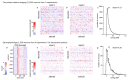
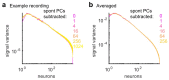
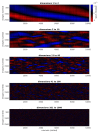
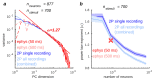
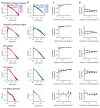
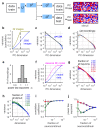
A neuronal population encodes information most efficiently when its stimulus responses are high-dimensional and uncorrelated, and most robustly when they are lower-dimensional and correlated. Here we analysed the dimensionality of the encoding of natural images by large populations of neurons in the visual cortex of awake mice. The evoked population activity was high-dimensional, and correlations obeyed an unexpected power law: the nth principal component variance scaled as 1/n. This scaling was not inherited from the power law spectrum of natural images, because it persisted after stimulus whitening. We proved mathematically that if the variance spectrum was to decay more slowly then the population code could not be smooth, allowing small changes in input to dominate population activity. The theory also predicts larger power-law exponents for lower-dimensional stimulus ensembles, which we validated experimentally. These results suggest that coding smoothness may represent a fundamental constraint that determines correlations in neural population codes.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Barlow HB. Possible principles underlying the transformations of sensory messages. In: Rosenblith W, editor. Sensory Communication. MIT Press; Cambridge, MA: 1961. pp. 217–234.
-
- Atick JJ, Redlich AN. Towards a theory of early visual processing. Neural Computation. 1990;2:308–320.
-
- Simoncelli EP, Olshausen BA. Natural image statistics and neural representation. Annual Review of Neuroscience. 2001;24:1193–1216. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

