Effect of belimumab on proteinuria and anti-phospholipase A2 receptor autoantibody in primary membranous nephropathy
- PMID: 31243451
- PMCID: PMC7139214
- DOI: 10.1093/ndt/gfz086
Effect of belimumab on proteinuria and anti-phospholipase A2 receptor autoantibody in primary membranous nephropathy
Abstract
Background: Immunosuppressant drugs reduce proteinuria and anti-phospholipase A2 receptor autoantibodies (PLA2R-Ab) in primary membranous nephropathy (PMN) with varying success and associated toxicities. This study aimed to evaluate the effect of belimumab on proteinuria and PLA2R-Ab in participants with PMN.
Methods: In this prospective, open-label, experimental medicine study, 14 participants with PMN and persistent nephrotic-range proteinuria received up to 2 years belimumab monotherapy (10 mg/kg, every 4 weeks). Changes in proteinuria (urinary protein:creatinine ratio), PLA2R-Ab, albumin, cholesterol, B-cell subsets and pharmacokinetics were analysed during treatment and up to 6 months after treatment.
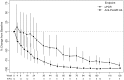
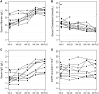
Results: Eleven participants completed to the primary endpoint (Week 28) and nine participants completed the study. In the intention-to-treat population population, baseline proteinuria of 724 mg/mmol [95% confidence interval (CI) 579-906] decreased to 498 mg/mmol (95% CI 383-649) and 130 mg/mmol (95% CI 54-312) at Weeks 28 and 104, respectively, with changes statistically significant from Week 36 (n = 11, P = 0.047). PLA2R-Ab decreased from 174 RU/mL (95% CI 79-384) at baseline to 46 RU/mL (95% CI 16-132) and 4 RU/mL (95% CI 2-6) at Weeks 28 and 104, respectively, becoming statistically significant by Week 12 (n = 13, P = 0.02). Nine participants achieved partial (n = 8) or complete (n = 1) remission. Participants with abnormal albumin and/or cholesterol at baseline gained normal/near normal levels by the last follow-up. Adverse events were consistent with those expected in this population.
Conclusions: Belimumab treatment in participants with PMN can reduce PLA2R-Ab and subsequently proteinuria, important preludes to remission induction.
Keywords: anti-phospholipase A2 receptor antibody; belimumab; pharmacokinetics; primary membranous nephropathy; proteinuria.
© The Author(s) 2019. Published by Oxford University Press on behalf of ERA-EDTA.
Figures

Comment in
-
Membranous glomerulonephritis: a step forward in B-cell targeting therapy?Nephrol Dial Transplant. 2020 Apr 1;35(4):549-551. doi: 10.1093/ndt/gfz139. Nephrol Dial Transplant. 2020. PMID: 31377779 No abstract available.
References
-
- McQuarrie EP, Stirling CM, Geddes CC.. Idiopathic membranous nephropathy and nephrotic syndrome: outcome in the era of evidence-based therapy. Nephrol Dial Transplant 2012; 27: 235–242 - PubMed
-
- Ponticelli C, Altieri P, Scolari F. et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 1998; 9: 444–450 - PubMed
-
- Ponticelli C, Zucchelli P, Passerini P. et al. A 10-year follow-up of a randomized study with methyiprednisolone and chlorambucil in membranous nephropathy. Kidney Int 1995; 48: 1600–1604 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

