Selective deletion of ENTPD1/CD39 in macrophages exacerbates biliary fibrosis in a mouse model of sclerosing cholangitis
- PMID: 31243614
- PMCID: PMC6737175
- DOI: 10.1007/s11302-019-09664-3
Selective deletion of ENTPD1/CD39 in macrophages exacerbates biliary fibrosis in a mouse model of sclerosing cholangitis
Abstract
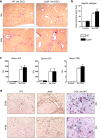
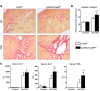
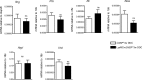
Purinergic signaling is important in the activation and differentiation of macrophages, which play divergent roles in the pathophysiology of liver fibrosis. The ectonucleotidase CD39 is known to modulate the immunoregulatory phenotype of macrophages, but whether this specifically impacts cholestatic liver injury is unknown. Here, we investigated the role of macrophage-expressed CD39 on the development of biliary injury and fibrosis in a mouse model of sclerosing cholangitis. Myeloid-specific CD39-deficient mice (LysMCreCd39fl/fl) were generated. Global CD39 null (Cd39-/-), wild-type (WT), LysMCreCd39fl/fl, and Cd39fl/fl control mice were exposed to 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) to induce biliary fibrosis. Hepatic hydroxyproline levels, liver histology, immunohistochemistry, mRNA expression levels, and serum biochemistry were then assessed. Following 3 weeks of DDC-feeding, Cd39-/- mice exhibited more severe fibrosis, when compared to WT mice as reflected by morphology and increased liver collagen content. Myeloid-specific CD39 deletion in LysMCreCd39fl/fl mice recapitulated the phenotype of global Cd39-/-, after exposure to DDC, and resulted in similar worsening of liver fibrosis when compared to Cd39fl/fl control animals. Further, DDC-treated LysMCreCd39fl/fl mice exhibited elevated serum levels of transaminases and total bilirubin, as well as increased hepatic expression of the profibrogenic genes Tgf-β1, Tnf-α, and α-Sma. However, no clear differences were observed in the expression of macrophage-elaborated specific cytokines between LysMCreCd39fl/fl and Cd39fl/fl animals subjected to biliary injury. Our results in the DDC-induced biliary type liver fibrosis model suggest that loss of CD39 expression on myeloid cells largely accounts for the exacerbated sclerosing cholangitis in global CD39 knockouts. These findings indicate that macrophage expressed CD39 protects from biliary liver injury and fibrosis and support a potential therapeutic target for human hepatobiliary diseases.
Keywords: CD39; Kupffer cells; Liver fibrosis; Primary sclerosing cholangitis; Purinergic signaling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
CsHscB Derived from a Liver Fluke Clonorchis sinensis Ameliorates Cholestatic Hepatic Fibrosis in a Mouse Model of Sclerosing Cholangitis.Curr Mol Med. 2024;24(4):505-515. doi: 10.2174/1566524023666230418111949. Curr Mol Med. 2024. PMID: 37076961
-
The ectonucleotidase ENTPD1/CD39 limits biliary injury and fibrosis in mouse models of sclerosing cholangitis.Hepatol Commun. 2017 Sep 26;1(9):957-972. doi: 10.1002/hep4.1084. eCollection 2017 Nov. Hepatol Commun. 2017. PMID: 29404503 Free PMC article.
-
Nrf2 Ameliorates DDC-Induced Sclerosing Cholangitis and Biliary Fibrosis and Improves the Regenerative Capacity of the Liver.Toxicol Sci. 2019 Jun 1;169(2):485-498. doi: 10.1093/toxsci/kfz055. Toxicol Sci. 2019. PMID: 30825315 Free PMC article.
-
Monoacylglycerol Lipase Inhibition Protects From Liver Injury in Mouse Models of Sclerosing Cholangitis.Hepatology. 2020 May;71(5):1750-1765. doi: 10.1002/hep.30929. Epub 2019 Dec 30. Hepatology. 2020. PMID: 31505038 Free PMC article.
-
Loss of hepatocyte Usp53 protects mice from a form of xenobiotic-induced liver injury.Biochim Biophys Acta Mol Basis Dis. 2025 Mar;1871(3):167624. doi: 10.1016/j.bbadis.2024.167624. Epub 2024 Dec 19. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39705897 Review.
Cited by
-
Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity.Nat Immunol. 2023 Feb;24(2):267-279. doi: 10.1038/s41590-022-01379-9. Epub 2022 Dec 21. Nat Immunol. 2023. PMID: 36543958 Free PMC article.
-
Purinergic and Adenosinergic Signaling in Pancreatobiliary Diseases.Front Physiol. 2022 Mar 14;13:849258. doi: 10.3389/fphys.2022.849258. eCollection 2022. Front Physiol. 2022. PMID: 35360246 Free PMC article. Review.
-
The Gut-Liver Axis in Chronic Liver Disease: A Macrophage Perspective.Cells. 2021 Oct 30;10(11):2959. doi: 10.3390/cells10112959. Cells. 2021. PMID: 34831182 Free PMC article. Review.
-
Ecto-Nucleotide Triphosphate Diphosphohydrolase-2 (NTPDase2) Deletion Increases Acetaminophen-Induced Hepatotoxicity.Int J Mol Sci. 2020 Aug 20;21(17):5998. doi: 10.3390/ijms21175998. Int J Mol Sci. 2020. PMID: 32825435 Free PMC article.
-
CD73 Inhibits cGAS-STING and Cooperates with CD39 to Promote Pancreatic Cancer.Cancer Immunol Res. 2023 Jan 3;11(1):56-71. doi: 10.1158/2326-6066.CIR-22-0260. Cancer Immunol Res. 2023. PMID: 36409930 Free PMC article.
References
-
- Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, Jaeschke H, Zatloukal K, Denk H, Trauner M. A new xenobiotic-induced mouse model of Sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

