Neurovascular Drug Biotransformation Machinery in Focal Human Epilepsies: Brain CYP3A4 Correlates with Seizure Frequency and Antiepileptic Drug Therapy
- PMID: 31243719
- PMCID: PMC6854685
- DOI: 10.1007/s12035-019-01673-y
Neurovascular Drug Biotransformation Machinery in Focal Human Epilepsies: Brain CYP3A4 Correlates with Seizure Frequency and Antiepileptic Drug Therapy
Abstract
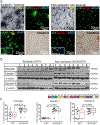
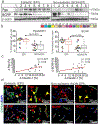
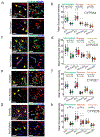
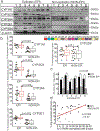
Pharmacoresistance is a major clinical challenge for approximately 30% of patients with epilepsy. Previous studies indicate nuclear receptors (NRs), drug efflux transporters, and cytochrome P450 enzymes (CYPs) control drug passage across the blood-brain barrier (BBB) in drug-resistant epilepsy. Here, we (1) evaluate BBB changes, neurovascular nuclear receptors, and drug transporters in lesional/epileptic (EPI) and non-lesional/non-epileptic (NON-EPI) regions of the same brain, (2) examine regional CYP expression and activity, and (3) investigate the association among CYP brain expression, seizure frequency, duration of epilepsy, and antiepileptic drug (AED) combination. We used surgically resected brain specimens from patients with medically intractable epilepsy (n = 22) where the epileptogenic loci were well-characterized by invasive and non-invasive methods; histology confirmed distinction of small NON-EPI regions from EPI tissues. NRs, transporters, CYPs, and tight-junction proteins were assessed by western blots/immunohistochemistry, and CYP metabolic activity was determined and compared. The relationship of CYP expression with seizure frequency, duration of epilepsy, and prescribed AEDs was evaluated. Decreased BBB tight-junction proteins accompanied IgG leakage in EPI regions and correlated with upregulated NR and efflux transporter levels. CYP expression and activity significantly increased in EPI compared to NON-EPI tissues. Change in EPI and NON-EPI CYP3A4 expression increased in patients taking AEDs that were CYP substrates, was downregulated when CYP- and non-CYP-substrate AEDs were given together, and correlated with seizure frequency. Our studies suggest focal neurovascular CYP-NR-transporter alterations, as demonstrated by the relationship of seizure frequency and AED combination to brain CYP3A4, might together impact biotransformation machinery of human pharmacoresistant epilepsy.
Keywords: Antiepileptic drugs; Blood-brain barrier; Cytochrome P450; Multiple drug transporters; Nuclear receptors; Pharmacoresistant epilepsy.
Conflict of interest statement
Disclosure
J.G.M. has conflict of interest with Zimmer Biomet, Cleveland. I.N. serves on the Speaker’ bureau and as a member of
Figures



References
-
- Kwan P (2011) Defining drug-resistant epilepsy. Neurology Asia 16: 67–69.
MeSH terms
Substances
Grants and funding
- R01NS078307/National Institute of Neurological Disorders and Stroke/National Institutes of Health
- R01 NS078307/NS/NINDS NIH HHS/United States
- ARDF1508CG/Alternatives Research & Development Foundation
- 18815/Brain and Behavior Research Foundation
- R01NS095825/National Institute of Neurological Disorders and Stroke/National Institutes of Health
LinkOut - more resources
Full Text Sources
Medical

