A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial
- PMID: 31243803
- PMCID: PMC6772897
- DOI: 10.1002/ajh.25564
A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial
Abstract
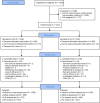
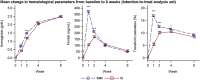
Iron deficiency anemia (IDA) is prevalent, and intravenous iron, especially if given in a single dose, may result in better adherence compared with oral iron. The present trial (FERWON-IDA) is part of the FERWON program with iron isomaltoside 1000/ferric derisomaltose (IIM), evaluating safety and efficacy of high dose IIM in IDA patients of mixed etiologies. This was a randomized, open-label, comparative, multi-center trial conducted in the USA. The IDA patients were randomized 2:1 to a single dose of 1000 mg IIM, or iron sucrose (IS) administered as 200 mg intravenous injections, up to five times. The co-primary endpoints were adjudicated serious or severe hypersensitivity reactions, and change in hemoglobin from baseline to week eight. A total of 1512 patients were enrolled. The frequency of patients with serious or severe hypersensitivity reactions was 0.3% (95% confidence interval: 0.06;0.88) vs 0.4% (0.05;1.45) in the IIM and IS group, respectively. The co-primary safety objective was met, and no risk difference was observed between groups. The co-primary efficacy endpoint of non-inferiority in hemoglobin change was met, and IIM led to a significantly more rapid hematological response in the first two weeks. The frequency of cardiovascular events was 0.8% and 1.2% in the IIM and IS group, respectively (P = .570). The frequency of hypophosphatemia was low in both groups. Iron isomaltoside administered as 1000 mg resulted in a more rapid and more pronounced hematological response, compared with IS, which required multiple visits. The safety profile was similar with a low frequency of hypersensitivity reactions and cardiovascular events.
© 2019 The Authors. American Journal of Hematology published by Wiley Periodicals, Inc.
Conflict of interest statement
Michael Auerbach receives research funding for data management from AMAG Pharmaceuticals.
David Henry has no conflict of interest.
Richard Derman has been a research and science consultant with Pharmacosmos and AMAG.
Lars L. Thomsen is employed by Pharmacosmos A/S.
Maureen M. Achebe has been on scientific advisory boards for Pharmacosmos and AMAG pharmaceuticals, and a consultant for Luitpold.
John Glaspy has been an advisor to AMAG Pharmaceuticals.
This work was funded by Pharmacosmos A/S and the investigators/institutions received a fee per patient.
Figures
References
-
- Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211‐222. - PubMed
-
- KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. 2012. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline.... Accessed May 22, 2019.
-
- Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545‐1553. - PubMed
-
- Macdougall IC, Bircher AJ, Eckardt KU, et al. Iron management in chronic kidney disease: conclusions from a “kidney disease: improving global outcomes” (KDIGO) controversies conference. Kidney Int. 2016;89:28‐39. - PubMed
-
- Macdougall IC, Vernon K. Complement activation‐related pseudo‐allergy: a fresh look at hypersensitivity reactions to intravenous iron. Am J Nephrol. 2017;45:60‐62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
