Bond-Forming and -Breaking Reactions at Sulfur(IV): Sulfoxides, Sulfonium Salts, Sulfur Ylides, and Sulfinate Salts
- PMID: 31243998
- PMCID: PMC6661881
- DOI: 10.1021/acs.chemrev.9b00111
Bond-Forming and -Breaking Reactions at Sulfur(IV): Sulfoxides, Sulfonium Salts, Sulfur Ylides, and Sulfinate Salts
Abstract
Organosulfur compounds have long played a vital role in organic chemistry and in the development of novel chemical structures and architectures. Prominent among these organosulfur compounds are those involving a sulfur(IV) center, which have been the subject of countless investigations over more than a hundred years. In addition to a long list of textbook sulfur-based reactions, there has been a sustained interest in the chemistry of organosulfur(IV) compounds in recent years. Of particular interest within organosulfur chemistry is the ease with which the synthetic chemist can effect a wide range of transformations through either bond formation or bond cleavage at sulfur. This review aims to cover the developments of the past decade in the chemistry of organic sulfur(IV) molecules and provide insight into both the wide range of reactions which critically rely on this versatile element and the diverse scaffolds that can thereby be synthesized.
Conflict of interest statement
The authors declare no competing financial interest.
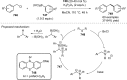
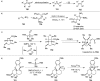
Figures
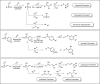
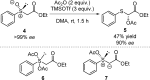
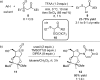
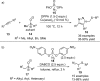


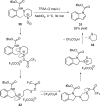
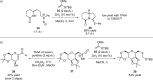
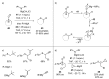

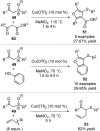
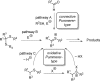

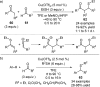


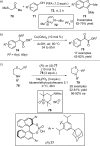
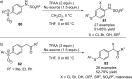




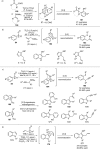

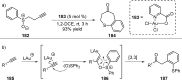
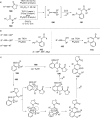

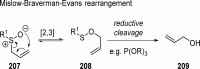






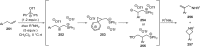
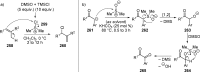
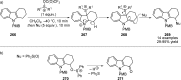
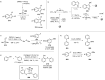
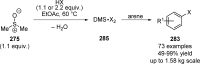
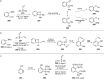


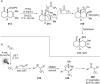
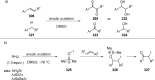
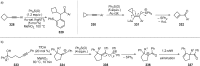

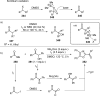


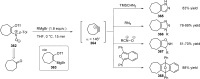
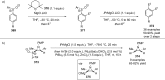






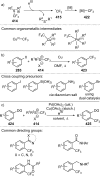
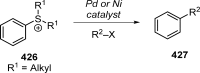


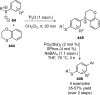
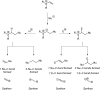
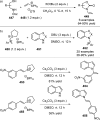
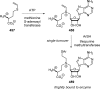
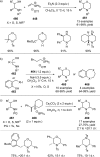


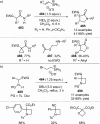


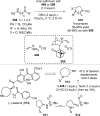
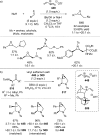
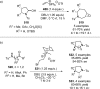
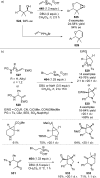

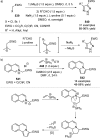

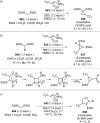


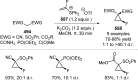

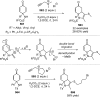
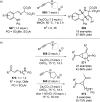
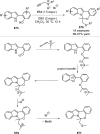



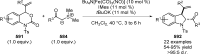

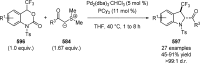




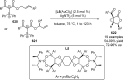




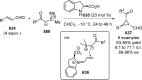
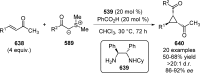

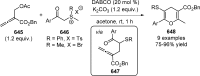






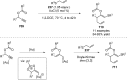
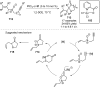
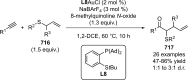
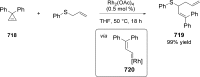

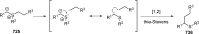
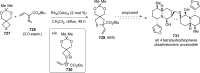
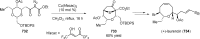


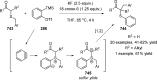



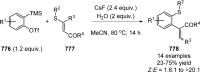
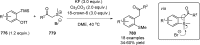


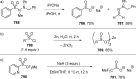
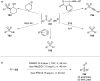









References
-
- De Lucci O.; Miotti U.; Modena G. The Pummerer Reaction of Sulfinyl Compounds. Organic Reactions 1991, 157–405. 10.1002/0471264180.or040.03. - DOI
-
- Akai S.; Kita Y. Recent Advances in Pummerer Reactions. Top. Curr. Chem. 2007, 274, 35–76. 10.1007/128_073. - DOI
-
- Hugenberg V.; Haufe G. Fluoro-Pummerer Rearrangement and Analogous Reactions. J. Fluorine Chem. 2012, 143, 238–262. 10.1016/j.jfluchem.2012.06.015. - DOI
-
- Gamba-Sánchez D.; Garzón-Posse F. Pummerer-Type Reactions as Powerful Tools in Organic Synthesis. Molecular Rearrangements in Organic Synthesis 2015, 661–702. 10.1002/9781118939901.ch20. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

