Cholesterol Biosynthesis and Uptake in Developing Neurons
- PMID: 31244054
- PMCID: PMC7184320
- DOI: 10.1021/acschemneuro.9b00248
Cholesterol Biosynthesis and Uptake in Developing Neurons
Abstract
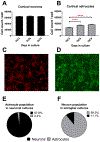
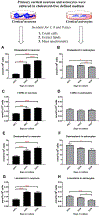
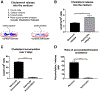
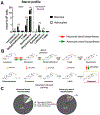
Brain cholesterol biosynthesis, a separate and distinct process from whole-body cholesterol homeostasis, starts during embryonic development. To gain a better understanding of the neuronal and glial contributions to the brain cholesterol pool, we studied this process in control, Dhcr7-/-, and Dhcr24-/- cell cultures. Our LC-MS/MS method allowed us to measure several different sterol intermediates and cholesterol during neuronal differentiation. We found that developing cortical neurons rely on endogenous cholesterol synthesis and utilize ApoE-complexed cholesterol and sterol precursors from their surroundings. Both developing neurons and astrocytes release cholesterol into their local environment. Our studies also uncovered that developing neurons produced significantly higher amounts of cholesterol per cell than the astrocytes. Finally, we established that both neurons and astroglia preferentially use the Bloch sterol biosynthesis pathway, where desmosterol is the immediate precursor to cholesterol. Overall, our studies suggest that endogenous sterol synthesis in developing neurons is a critical and complexly regulated homeostatic process during brain development.
Keywords: Dhcr24; Dhcr7; LC-MS/MS; Sterol biosynthesis; astrocytes; cholesterol; desmosterol; developing neurons.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
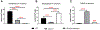
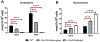


References
-
- Dietschy JM, and Turley SD (2001) Cholesterol metabolism in the brain. Curr. Opin. Lipidol 12, 105–12. - PubMed
-
- Dietschy JM, and Turley SD (2004) Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res 45, 1375–97. - PubMed
-
- Porter FD (2000) RSH/Smith-Lemli-Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol. Genet. Metab 71, 163–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

