Off-Pathway Assembly: A Broad-Spectrum Mechanism of Action for Drugs That Undermine Controlled HIV-1 Viral Capsid Formation
- PMID: 31244184
- PMCID: PMC6739737
- DOI: 10.1021/jacs.9b01413
Off-Pathway Assembly: A Broad-Spectrum Mechanism of Action for Drugs That Undermine Controlled HIV-1 Viral Capsid Formation
Abstract
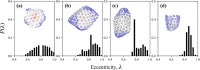
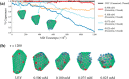
The early and late stages of human immunodeficiency virus (HIV) replication are orchestrated by the capsid (CA) protein, which self-assembles into a conical protein shell during viral maturation. Small molecule drugs known as capsid inhibitors (CIs) impede the highly regulated activity of CA. Intriguingly, a few CIs, such as PF-3450074 (PF74) and GS-CA1, exhibit effects at multiple stages of the viral lifecycle at effective concentrations in the pM to nM regimes, while the majority of CIs target a single stage of the viral lifecycle and are effective at nM to μM concentrations. In this work, we use coarse-grained molecular dynamics simulations to elucidate the molecular mechanisms that enable CIs to have such curious broad-spectrum activity. Our quantitatively analyzed findings show that CIs can have a profound impact on the hierarchical self-assembly of CA by perturbing populations of small CA oligomers. The self-assembly process is accelerated by the emergence of alternative assembly pathways that favor the rapid incorporation of CA pentamers, and leads to increased structural pleomorphism in mature capsids. Two relevant phenotypes are observed: (1) eccentric capsid formation that may fail to encase the viral genome and (2) rapid disassembly of the capsid, which express at late and early stages of infection, respectively. Finally, our study emphasizes the importance of adopting a dynamical perspective on inhibitory mechanisms and provides a basis for the design of future therapeutics that are effective at low stoichiometric ratios of drug to protein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

