Effects of Moisture Contents on Shale Gas Recovery and CO2 Sequestration
- PMID: 31244260
- PMCID: PMC7007254
- DOI: 10.1021/acs.langmuir.9b00862
Effects of Moisture Contents on Shale Gas Recovery and CO2 Sequestration
Abstract
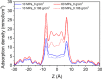
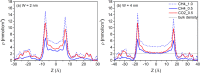
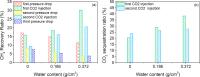
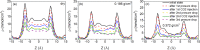
Enhanced recovery of shale gas with CO2 injection has attracted extensive attention as it combines the advantages of improved efficiency of shale gas recovery and reduced greenhouse gas emissions via CO2 geological sequestration. On the other hand, the microscopic mechanism of enhanced shale gas recovery with CO2 injection and the influence of the subsurface water confined in the shale nanopores remain ambiguous. Here, we use grand canonical Monte Carlo (GCMC) simulations to investigate the effect of moisture on the shale gas recovery and CO2 sequestration by calculating the adsorption of CH4 and CO2 in dry and moist kerogen slit pores. Simulation results indicate that water accumulates in the form of clusters in the middle of the kerogen slit pore. Formation of water clusters in kerogen slit pores reduces pore filling by methane molecules, resulting in a decrease in the methane sorption capacity. For the sorption of CH4/CO2 binary mixtures in kerogen slit pores, the CH4 sorption capacity decreases as the moisture content increases, whereas the effect of moisture on CO2 sorption capacity is related to its mole fraction in the CH4/CO2 binary mixture. Furthermore, we propose a reference route for shale gas recovery and find that the pressure drawdown and CO2 injection exhibit different mechanisms for gas recovery. Pressure drawdown mainly extracts the CH4 molecules distributed in the middle of kerogen slit pores, while CO2 injection recovers CH4 molecules from the adsorption layer. When the water content increases, the recovery ratio of the pressure drawdown declines, while that of CO2 injection increases, especially in the first stage of CO2 injection. The CO2 sequestration efficiency is higher under higher water content. These findings provide the theoretical foundation for optimization of the shale gas recovery process, as well as effective CO2 sequestration in depleted gas reservoirs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Melikoglu M. Shale Gas: Analysis of Its Role in the Global Energy Market. Renewable Sustainable Energy Rev. 2014, 37, 460–468. 10.1016/j.rser.2014.05.002. - DOI
-
- Arogundade O.; Sohrabi M.. A Review of Recent Developments and Challenges in Shale Gas Recovery, SPE Saudi Arabia Section Technical Symposium and Exhibition, 8–11 April 2012, Al-Khobar, Saudi Arabia, 2012.
-
- Middleton R.; Viswanathan H.; Currier R.; Gupta R. CO2 as a Fracturing Fluid: Potential for Commercial-Scale Shale Gas Production and CO2 Sequestration. Energy Procedia 2014, 63, 7780–7784. 10.1016/j.egypro.2014.11.812. - DOI
LinkOut - more resources
Full Text Sources

