B Cell-Based Cancer Immunotherapy
- PMID: 31244580
- PMCID: PMC6558332
- DOI: 10.1159/000496166
B Cell-Based Cancer Immunotherapy
Abstract
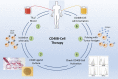
B cells are not only producers of antibodies, but also contribute to immune regulation or act as potent antigen-presenting cells. The potential of B cells for cellular therapy is still largely underestimated, despite their multiple diverse effector functions. The CD40L/CD40 signaling pathway is the most potent activator of antigen presentation capacity in B lymphocytes. CD40-activated B cells are potent antigen-presenting cells that induce specific T-cell responses in vitro and in vivo. In preclinical cancer models in mice and dogs, CD40-activated B cell-based cancer immunotherapy was able to induce effective antitumor immunity. So far, there have been only few early-stage clinical studies involving B cell-based cancer vaccines. These trials indicate that B cell-based immunotherapy is generally safe and associated with little toxicity. Furthermore, these studies suggest that B-cell immunotherapy can elicit antitumor T-cell responses. Alongside the recent advances in cellular therapies in general, major obstacles for generation of good manufacturing practice-manufactured B-cell immunotherapies have been overcome. Thus, a first clinical trial involving CD40-activated B cells might be in reach.
Keywords: Antigen presentation; B-cell therapy; Cellular therapy.
Figures
Similar articles
-
RNA-loaded CD40-activated B cells stimulate antigen-specific T-cell responses in dogs with spontaneous lymphoma.Gene Ther. 2008 Jul;15(13):955-65. doi: 10.1038/gt.2008.22. Epub 2008 Mar 13. Gene Ther. 2008. PMID: 18337841
-
A multimerized form of recombinant human CD40 ligand supports long-term activation and proliferation of B cells.Cytotherapy. 2014 Nov;16(11):1537-1544. doi: 10.1016/j.jcyt.2014.05.011. Cytotherapy. 2014. PMID: 25287602
-
CD40-activated B-cell chronic lymphocytic leukemia cells for tumor immunotherapy: stimulation of allogeneic versus autologous T cells generates different types of effector cells.Blood. 1999 Mar 15;93(6):1992-2002. Blood. 1999. PMID: 10068672
-
The role of CD40 ligand in costimulation and T-cell activation.Immunol Rev. 1996 Oct;153:85-106. doi: 10.1111/j.1600-065x.1996.tb00921.x. Immunol Rev. 1996. PMID: 9010720 Review.
-
Design of CD40 agonists and their use in growing B cells for cancer immunotherapy.Int Rev Immunol. 2012 Aug;31(4):279-88. doi: 10.3109/08830185.2012.703272. Int Rev Immunol. 2012. PMID: 22804572 Free PMC article. Review.
Cited by
-
XRCC1: a potential prognostic and immunological biomarker in LGG based on systematic pan-cancer analysis.Aging (Albany NY). 2024 Jan 12;16(1):872-910. doi: 10.18632/aging.205426. Epub 2024 Jan 12. Aging (Albany NY). 2024. PMID: 38217545 Free PMC article.
-
Efficient adoptive transfer of autologous modified B cells: a new humanized platform mouse model for testing B cells reprogramming therapies.Cancer Immunol Immunother. 2022 Jul;71(7):1771-1775. doi: 10.1007/s00262-021-03101-4. Epub 2021 Nov 8. Cancer Immunol Immunother. 2022. PMID: 34748076 Free PMC article.
-
Dysregulation of TFH-B-TRM lymphocyte cooperation is associated with unfavorable anti-PD-1 responses in EGFR-mutant lung cancer.Nat Commun. 2021 Oct 18;12(1):6068. doi: 10.1038/s41467-021-26362-0. Nat Commun. 2021. PMID: 34663810 Free PMC article.
-
DNA vaccines for SARS-CoV-2: toward third-generation vaccination era.Expert Rev Vaccines. 2021 Dec;20(12):1549-1560. doi: 10.1080/14760584.2021.1987223. Epub 2021 Oct 28. Expert Rev Vaccines. 2021. PMID: 34582298 Free PMC article. Review.
-
Next-Generation Immunotherapies to Improve Anticancer Immunity.Front Pharmacol. 2021 Jan 11;11:566401. doi: 10.3389/fphar.2020.566401. eCollection 2020. Front Pharmacol. 2021. PMID: 33505304 Free PMC article. Review.
References
-
- Karpusas M, Hsu YM, Wang JH, Thompson J, Lederman S, Chess L, et al. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure. 1995 Oct;3((10)):1031–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

