Heterozygous Mylk3 Knockout Mice Partially Recapitulate Human DCM With Heterozygous MYLK3 Mutations
- PMID: 31244672
- PMCID: PMC6563786
- DOI: 10.3389/fphys.2019.00696
Heterozygous Mylk3 Knockout Mice Partially Recapitulate Human DCM With Heterozygous MYLK3 Mutations
Abstract
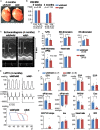
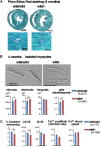
Backgrounds: Recent studies identified heterozygous variants in MYLK3 gene that encodes cardiac myosin light chain kinase (cMLCK) are related to familial dilated cardiomyopathy (DCM) for the first time. Autosomal dominant traits suggest that pathogenesis of DCM could be related to heterozygous MYLK3 loss-of-function variants (haploinsufficiency). We previously generated and examined homozygous Mylk3 knockout mice that lead to heart failure. It had yet to be examined whether heterozygous Mylk3 knockout mice represent a DCM-like phenotype. Methods and Results: Heterozygous knockout (Mylk3 wild/-) mice were examined regarding cardiac function, heart histology and expression of cMLCK protein and mRNA relative to age-matched wild-type controls (Mylk3 wild/wild). At 4 months of age, cardiac contractility in heterozygous knockout mice was reduced with percent fractional shortening of 23.3 ± 1.2% compared to 30.1 ± 1.8% in control (Mylk3 wild/- vs. Mylk3 wild/wild, n = 9 each). In 4-month-old heterozygous knockout hearts, expression of cMLCK mRNA was expectedly reduced by almost half, however, protein expression was reduced by approximately 75% relative to the control wild-type (Mylk3 wild/- vs. Mylk3 wild/wild, n = 9 each). Isolated ventricular cardiomyocytes from heterozygous knockout mice were larger with increase of short-axis length relative to the cardiomyocytes from control mice. However, increase of heart failure markers as well as interstitial fibrosis were not evident in heterozygous knockout mice compared to controls. Conclusion: Heterozygous Mylk3 knockout mice show mild reduction of cardiac contractility by 4 months of age, and proteins reduced by approximately 75% relative to the control wild-type mice. These mice partly resemble human with the heterozygous MYLK3 mutation, but the reduction in cardiac contractility was milder.
Keywords: animal model of human disease; genetic dilated cardiomyopathy; heart; heterozygous knockout; kinase.
Figures


Similar articles
-
Acute heart failure with cardiomyocyte atrophy induced in adult mice by ablation of cardiac myosin light chain kinase.Cardiovasc Res. 2016 Jul 1;111(1):34-43. doi: 10.1093/cvr/cvw069. Epub 2016 Mar 29. Cardiovasc Res. 2016. PMID: 27025239 Free PMC article.
-
Restoration of Cardiac Myosin Light Chain Kinase Ameliorates Systolic Dysfunction by Reducing Superrelaxed Myosin.Circulation. 2023 Jun 20;147(25):1902-1918. doi: 10.1161/CIRCULATIONAHA.122.062885. Epub 2023 May 2. Circulation. 2023. PMID: 37128901 Free PMC article.
-
Heart Failure Induced by Perinatal Ablation of Cardiac Myosin Light Chain Kinase.Front Physiol. 2016 Oct 26;7:480. doi: 10.3389/fphys.2016.00480. eCollection 2016. Front Physiol. 2016. PMID: 27833563 Free PMC article.
-
Impact of cardiac myosin light chain kinase gene mutation on development of dilated cardiomyopathy.ESC Heart Fail. 2019 Apr;6(2):406-415. doi: 10.1002/ehf2.12410. Epub 2019 Jan 28. ESC Heart Fail. 2019. PMID: 30690923 Free PMC article.
-
Identification of MYLK3 mutations in familial dilated cardiomyopathy.Sci Rep. 2017 Dec 13;7(1):17495. doi: 10.1038/s41598-017-17769-1. Sci Rep. 2017. PMID: 29235529 Free PMC article.
Cited by
-
A missense mutation in the RSRSP stretch of Rbm20 causes dilated cardiomyopathy and atrial fibrillation in mice.Sci Rep. 2020 Oct 27;10(1):17894. doi: 10.1038/s41598-020-74800-8. Sci Rep. 2020. PMID: 33110103 Free PMC article.
-
Myofilament dysfunction in diastolic heart failure.Heart Fail Rev. 2024 Jan;29(1):79-93. doi: 10.1007/s10741-023-10352-z. Epub 2023 Oct 14. Heart Fail Rev. 2024. PMID: 37837495 Free PMC article. Review.
-
Retrospective Study of Intercalated Disk Defects Associated with Dilated Cardiomyopathy, Atrial Thrombosis, and Heart Failure in BALB/c Mice Deficient in IL4 Receptor α.Comp Med. 2020 Jun 1;70(3):266-276. doi: 10.30802/AALAS-CM-19-000059. Epub 2020 May 8. Comp Med. 2020. PMID: 32384942 Free PMC article.
References
-
- Davis J. S., Hassanzadeh S., Winitsky S., Lin H., Satorius C., Vemuri R., et al. (2001). The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell 107 631–641. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

