The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model
- PMID: 31244683
- PMCID: PMC6581701
- DOI: 10.3389/fphys.2019.00729
The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model
Abstract
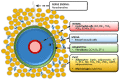
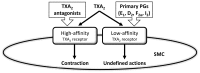
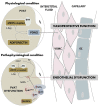
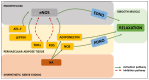
Our understanding of the regulation of vascular function, specifically that of vasomotion, has evolved dramatically over the past few decades. The classic conception of a vascular system solely regulated by circulating hormones and sympathetic innervation gave way to a vision of a local regulation. Initially by the so-called, autacoids like prostacyclin, which represented the first endothelium-derived paracrine regulator of smooth muscle. This was the prelude of the EDRF-nitric oxide age that has occupied vascular scientists for nearly 30 years. Endothelial cells revealed to have the ability to generate numerous mediators besides prostacyclin and nitric oxide (NO). The need to classify these substances led to the coining of the terms: endothelium-derived relaxing, hyperpolarizing and contracting factors, which included various prostaglandins, thromboxane A2, endothelin, as well numerous candidates for the hyperpolarizing factor. The opposite layer of the vascular wall, the adventitia, eventually and for a quite short period of time, enjoyed the attention of some vascular physiologists. Adventitial fibroblasts were recognized as paracrine cells to the smooth muscle because of their ability to produce some substances such as superoxide. Remarkably, this took place before our awareness of the functional potential of another adventitial cell, the adipocyte. Possibly, because the perivascular adipose tissue (PVAT) was systematically removed during the experiments as considered a non-vascular artifact tissue, it took quite long to be considered a major source of paracrine substances. These are now being integrated in the vast pool of mediators synthesized by adipocytes, known as adipokines. They include hormones involved in metabolic regulation, like leptin or adiponectin; classic vascular mediators like NO, angiotensin II or catecholamines; and inflammatory mediators or adipocytokines. The first substance studied was an anti-contractile factor named adipose-derived relaxing factor of uncertain chemical nature but possibly, some of the relaxing mediators mentioned above are behind this factor. This manuscript intends to review the vascular regulation from the point of view of the paracrine control exerted by the cells present in the vascular environment, namely, endothelial, adventitial, adipocyte and vascular stromal cells.
Keywords: EDCF; EDHF; PVAT-derived NO; adventitia; endothelium-derived NO; perivascular adipose tissue; prostaglandins.
Figures
References
-
- Altiere R. J., Kiritsy-Roy J. A., Catravas J. D. (1986). Acetylcholine-induced contractions in isolated rabbit pulmonary arteries: role of thromboxane A2. J. Pharmacol. Exp. Ther. 236 535–541. - PubMed
-
- An S. J., Boyd R., Wang Y., Qiu X., Wang H. D. (2006). Endothelin-1 expression in vascular adventitial fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 290 H700–H708. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

