Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function
- PMID: 31244820
- PMCID: PMC6562565
- DOI: 10.3389/fimmu.2019.00925
Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function
Abstract
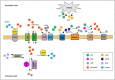
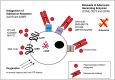
T cells play a critical role in cancer control, but a range of potent immunosuppressive mechanisms can be upregulated in the tumor microenvironment (TME) to abrogate their activity. While various immunotherapies (IMTs) aiming at re-invigorating the T-cell-mediated anti-tumor response, such as immune checkpoint blockade (ICB), and the adoptive cell transfer (ACT) of natural or gene-engineered ex vivo expanded tumor-specific T cells, have led to unprecedented clinical responses, only a small proportion of cancer patients benefit from these treatments. Important research efforts are thus underway to identify biomarkers of response, as well as to develop personalized combinatorial approaches that can target other inhibitory mechanisms at play in the TME. In recent years, adenosinergic signaling has emerged as a powerful immuno-metabolic checkpoint in tumors. Like several other barriers in the TME, such as the PD-1/PDL-1 axis, CTLA-4, and indoleamine 2,3-dioxygenase (IDO-1), adenosine plays important physiologic roles, but has been co-opted by tumors to promote their growth and impair immunity. Several agents counteracting the adenosine axis have been developed, and pre-clinical studies have demonstrated important anti-tumor activity, alone and in combination with other IMTs including ICB and ACT. Here we review the regulation of adenosine levels and mechanisms by which it promotes tumor growth and broadly suppresses protective immunity, with extra focus on the attenuation of T cell function. Finally, we present an overview of promising pre-clinical and clinical approaches being explored for blocking the adenosine axis for enhanced control of solid tumors.
Keywords: CD39; CD73; T cells; adenosine; cAMP; cancer immunotherapy; tumor microenvironment.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

