Identification and in vivo Efficacy Assessment of Approved Orally Bioavailable Human Host Protein-Targeting Drugs With Broad Anti-influenza A Activity
- PMID: 31244822
- PMCID: PMC6563844
- DOI: 10.3389/fimmu.2019.01097
Identification and in vivo Efficacy Assessment of Approved Orally Bioavailable Human Host Protein-Targeting Drugs With Broad Anti-influenza A Activity
Abstract
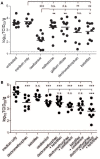
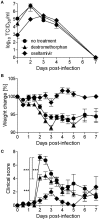
The high genetic variability of influenza A viruses poses a continual challenge to seasonal and pandemic vaccine development, leaving antiviral drugs as the first line of defense against antigenically different strains or new subtypes. As resistance against drugs targeting viral proteins emerges rapidly, we assessed the antiviral activity of already approved drugs that target cellular proteins involved in the viral life cycle and were orally bioavailable. Out of 15 candidate compounds, four were able to inhibit infection by 10- to 100-fold without causing toxicity, in vitro. Two of the drugs, dextromethorphan and ketotifen, displayed a 50% effective dose between 5 and 50 μM, not only for the classic H1N1 PR8 strain, but also for a pandemic H1N1 and a seasonal H3N2 strain. Efficacy assessment in mice revealed that dextromethorphan consistently resulted in a significant reduction of viral lung titers and also enhanced the efficacy of oseltamivir. Dextromethorphan treatment of ferrets infected with a pandemic H1N1 strain led to a reduction in clinical disease severity, but no effect on viral titer was observed. In addition to identifying dextromethorphan as a potential influenza treatment option, our study illustrates the feasibility of a bioinformatics-driven rational approach for repurposing approved drugs against infectious diseases.
Keywords: animal models; antiviral (H1N1 and H3N2) activity; drug repurposing; host protein-targeting drugs; influenza A virus.
Figures

References
-
- Shaw ML, Palese P. Orthomyxoviridae. In: Knipe DM, Howley PM. editors. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; (2013). p. 1151–85.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

