Dermatitis Herpetiformis: Novel Perspectives
- PMID: 31244841
- PMCID: PMC6579917
- DOI: 10.3389/fimmu.2019.01290
Dermatitis Herpetiformis: Novel Perspectives
Abstract
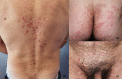
Dermatitis herpetiformis (DH) is an inflammatory disease of the skin, considered the specific cutaneous manifestation of celiac disease (CD). Both DH and CD occur in gluten-sensitive individuals, share the same Human Leukocyte Antigen (HLA) haplotypes (DQ2 and DQ8), and improve following the administration of a gluten-free diet. Moreover, almost all DH patients show typical CD alterations at the small bowel biopsy, ranging from villous atrophy to augmented presence of intraepithelial lymphocytes, as well as the generation of circulating autoantibodies against tissue transglutaminase (tTG). Clinically, DH presents with polymorphic lesions, including papules, vesicles, and small blisters, symmetrically distributed in typical anatomical sites including the extensor aspects of the limbs, the elbows, the sacral regions, and the buttocks. Intense pruritus is almost the rule. However, many atypical presentations of DH have also been reported. Moreover, recent evidence suggested that DH is changing. Firstly, some studies reported a reduced incidence of DH, probably due to early recognition of CD, so that there is not enough time for DH to develop. Moreover, data from Japanese literature highlighted the absence of intestinal involvement as well as of the typical serological markers of CD (i.e., anti-tTG antibodies) in Japanese patients with DH. Similar cases may also occur in Caucasian patients, complicating DH diagnosis. The latter relies on the combination of clinical, histopathologic, and immunopathologic findings. Detecting granular IgA deposits at the dermal-epidermal junction by direct immunofluorescence (DIF) from perilesional skin represents the most specific diagnostic tool. Further, assessing serum titers of autoantibodies against epidermal transglutaminase (eTG), the supposed autoantigen of DH, may also serve as a clue for the diagnosis. However, a study from our group has recently demonstrated that granular IgA deposits may also occur in celiac patients with non-DH inflammatory skin diseases, raising questions about the effective role of eTG IgA autoantibodies in DH and suggesting the need of revising diagnostic criteria, conceivably emphasizing clinical aspects of the disease along with DIF. DH usually responds to the gluten-free diet. Topical clobetasol ointment or dapsone may be also applied to favor rapid disease control. Our review will focus on novel pathogenic insights, controversies, and management aspects of DH.
Keywords: coeliac disease; dermatitis herpetiformis; direct immunofluorescence; epidermal transglutaminase; non-coeliac gluten sensitivity.
Figures



References
-
- Esteves J, Brandao FN. Effect of sulfonamides and sulfones on Duhring's disease. Clin Lat. (1952) 2:34–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

