Engineered mosaic protein polymers; a simple route to multifunctional biomaterials
- PMID: 31244892
- PMCID: PMC6582577
- DOI: 10.1186/s13036-019-0183-2
Engineered mosaic protein polymers; a simple route to multifunctional biomaterials
Abstract
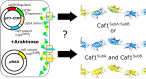
Background: Engineered living materials (ELMs) are an exciting new frontier, where living organisms create highly functional materials. In particular, protein ELMs have the advantage that their properties can be manipulated via simple molecular biology. Caf1 is a protein ELM that is especially attractive as a biomaterial on account of its unique combination of properties: bacterial cells export it as a massive, modular, non-covalent polymer which is resistant to thermal and chemical degradation and free from animal material. Moreover, it is biologically inert, allowing the bioactivity of each 15 kDa monomeric Caf1 subunit to be specifically engineered by mutagenesis and co-expressed in the same Escherichia coli cell to produce a mixture of bioactive Caf1 subunits.
Results: Here, we show by gel electrophoresis and transmission electron microscopy that the bacterial cells combine these subunits into true mosaic heteropolymers. By combining two separate bioactive motifs in a single mosaic polymer we demonstrate its utility by stimulating the early stages of bone formation by primary human bone marrow stromal cells. Finally, using a synthetic biology approach, we engineer a mosaic of three components, demonstrating that Caf1 complexity depends solely upon the variety of monomers available.
Conclusions: These results demonstrate the utility of engineered Caf1 mosaic polymers as a simple route towards the production of multifunctional biomaterials that will be useful in biomedical applications such as 3D tissue culture and wound healing. Additionally, in situ Caf1 producing cells could create complex bacterial communities for biotechnology.
Keywords: Biomaterials; Bone; Electron microscopy; Protein engineering; Synthetic biology; Tissue scaffolds.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures





Similar articles
-
The polymer and materials science of the bacterial fimbriae Caf1.Biomater Sci. 2023 Nov 7;11(22):7229-7246. doi: 10.1039/d3bm01075a. Biomater Sci. 2023. PMID: 37791425 Free PMC article. Review.
-
Thermal stability and rheological properties of the 'non-stick' Caf1 biomaterial.Biomed Mater. 2017 Sep 13;12(5):051001. doi: 10.1088/1748-605X/aa7a89. Biomed Mater. 2017. PMID: 28632140
-
Tuneable hydrogels of Caf1 protein fibers.Mater Sci Eng C Mater Biol Appl. 2018 Dec 1;93:88-95. doi: 10.1016/j.msec.2018.07.063. Epub 2018 Jul 24. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 30274124
-
Hydrogels of engineered bacterial fimbriae can finely tune 2D human cell culture.Biomater Sci. 2021 Apr 7;9(7):2542-2552. doi: 10.1039/d0bm01966f. Epub 2021 Feb 11. Biomater Sci. 2021. PMID: 33571331
-
Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration.Acta Biomater. 2021 Dec;136:1-36. doi: 10.1016/j.actbio.2021.09.034. Epub 2021 Sep 23. Acta Biomater. 2021. PMID: 34562661 Review.
Cited by
-
Unraveling the molecular determinants of the anti-phagocytic protein cloak of plague bacteria.PLoS Pathog. 2022 Mar 31;18(3):e1010447. doi: 10.1371/journal.ppat.1010447. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35358289 Free PMC article.
-
Spatial-Controlled Coating of Pro-Angiogenic Proteins on 3D Porous Hydrogels Guides Endothelial Cell Behavior.Int J Mol Sci. 2022 Nov 23;23(23):14604. doi: 10.3390/ijms232314604. Int J Mol Sci. 2022. PMID: 36498931 Free PMC article.
-
Advances in nanotechnology for targeting cancer-associated fibroblasts: A review of multi-strategy drug delivery and preclinical insights.APL Bioeng. 2025 Mar 13;9(1):011502. doi: 10.1063/5.0244706. eCollection 2025 Mar. APL Bioeng. 2025. PMID: 40094065 Free PMC article. Review.
-
Exploiting Meltable Protein Hydrogels to Encapsulate and Culture Cells in 3D.Macromol Biosci. 2022 Sep;22(9):e2200134. doi: 10.1002/mabi.202200134. Epub 2022 Jul 13. Macromol Biosci. 2022. PMID: 35780498 Free PMC article.
-
The polymer and materials science of the bacterial fimbriae Caf1.Biomater Sci. 2023 Nov 7;11(22):7229-7246. doi: 10.1039/d3bm01075a. Biomater Sci. 2023. PMID: 37791425 Free PMC article. Review.
References
-
- Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3(3):1863–1887. doi: 10.3390/ma3031863. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

