Pathophysiology and management of diabetic gastroenteropathy
- PMID: 31244895
- PMCID: PMC6580709
- DOI: 10.1177/1756284819852047
Pathophysiology and management of diabetic gastroenteropathy
Abstract
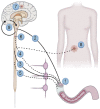
Polyneuropathy is a common complication to diabetes. Neuropathies within the enteric nervous system are associated with gastroenteropathy and marked symptoms that severely reduce quality of life. Symptoms are pleomorphic but include nausea, vomiting, dysphagia, dyspepsia, pain, bloating, diarrhoea, constipation and faecal incontinence. The aims of this review are fourfold. First, to provide a summary of the pathophysiology underlying diabetic gastroenteropathy. Secondly to give an overview of the diagnostic methods. Thirdly, to provide clinicians with a focussed overview of current and future methods for pharmacological and nonpharmacological treatment modalities. Pharmacological management is categorised according to symptoms arising from the upper or lower gut as well as sensory dysfunctions. Dietary management is central to improvement of symptoms and is discussed in detail, and neuromodulatory treatment modalities and other emerging management strategies for diabetic gastroenteropathy are discussed. Finally, we propose a diagnostic/investigation algorithm that can be used to support multidisciplinary management.
Keywords: complications; diabetes mellitus; diabetic neuropathies; enteric nervous system; enteropathy; gastrointestinal motility; gastrointestinal transit; pharmacology.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures


References
-
- Terkelsen AJ, Karlsson P, Lauria G, et al. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 2017; 16: 934–944. - PubMed
-
- Breiner A, Lovblom LE, Perkins BA, et al. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014; 37: 1418–1424. - PubMed
-
- Spångéus A, El-Salhy M, Suhr O, et al. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol 1999; 34: 1196–202. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

