Integrating 3D Cell Culture of PC12 Cells with Microchip-Based Electrochemical Detection
- PMID: 31244918
- PMCID: PMC6594695
- DOI: 10.1039/C8AY02672F
Integrating 3D Cell Culture of PC12 Cells with Microchip-Based Electrochemical Detection
Abstract
Developing in vitro cell culture models that accurately mimic in vivo processes in a manner that also enables near real-time analysis of neurotransmitters is an important research area. New technologies being developed such as 3D scaffolds for cell culture and 3D printed microfluidics provide an opportunity for such advancements. In this work, PC12 cells were used as a model system and they were immobilized onto a 3D scaffold of polystyrene (PS) fibers. These fibers were created by electrospinning onto PS sheets, which were laser cut and, after cell seeding, inserted into a 3D printed microfluidic device. The 3D printed device was designed with threads for connecting commercial fittings (to integrate automated pumps and a 4-port injection system) and a steel pin for simple coupling with PDMS/polystyrene analytical devices. A straight PDMS channel was used for simple (and continuous) flow-based detection by sealing onto a PS base containing an embedded gold array working electrode and a platinum pseudo-reference. Electrochemical detection of stimulated catecholamine release was demonstrated. The insert-based system was then integrated with a bilayer valving PDMS device (for microchip electrophoresis) sealed onto a PS base (with electrodes for electrochemical detection). This base was embedded with a Pd decoupler (for grounding the separation voltage and adsorbing hydrogen) and a 33 µm carbon fiber working electrode for in-channel detection. PC12 cells were stimulated in the 3D cell culture device, and the valving/electrophoresis microchip was able to separate and detect dopamine and norepinephrine release. This work demonstrates the ability to integrate 3D cell scaffolds with microchip-based analysis for detection of multiple analytes released from cells.
Conflict of interest statement
Conflicts of interest There are no conflicts of interest to declare.
Figures


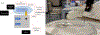
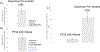
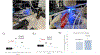
Similar articles
-
Integrated hybrid polystyrene-polydimethylsiloxane device for monitoring cellular release with microchip electrophoresis and electrochemical detection.Anal Methods. 2015 Feb 7;7(3):884-893. doi: 10.1039/C4AY02569E. Anal Methods. 2015. PMID: 25663849 Free PMC article.
-
Enhanced Microchip Electrophoresis Separations Combined with Electrochemical Detection Utilizing a Capillary Embedded in Polystyrene.Anal Methods. 2018 Jan 7;10(1):37-45. doi: 10.1039/C7AY02505J. Epub 2017 Dec 6. Anal Methods. 2018. PMID: 29707044 Free PMC article.
-
Integration of microchip electrophoresis with electrochemical detection using an epoxy-based molding method to embed multiple electrode materials.Electrophoresis. 2011 Nov;32(22):3121-8. doi: 10.1002/elps.201100433. Epub 2011 Oct 31. Electrophoresis. 2011. PMID: 22038707 Free PMC article.
-
Performance evaluation of a capillary electrophoresis electrochemical chip integrated with gold nanoelectrode ensemble working and decoupler electrodes.J Chromatogr A. 2008 Jun 20;1194(2):231-6. doi: 10.1016/j.chroma.2008.04.056. Epub 2008 Apr 25. J Chromatogr A. 2008. PMID: 18485353
-
Use of epoxy-embedded electrodes to integrate electrochemical detection with microchip-based analysis systems.Electrophoresis. 2011 Apr;32(8):822-31. doi: 10.1002/elps.201000665. Epub 2011 Mar 17. Electrophoresis. 2011. PMID: 21413031 Free PMC article. Review.
Cited by
-
Biological applications of microchip electrophoresis with amperometric detection: in vivo monitoring and cell analysis.Anal Bioanal Chem. 2020 Sep;412(24):6101-6119. doi: 10.1007/s00216-020-02647-z. Epub 2020 Apr 28. Anal Bioanal Chem. 2020. PMID: 32347360 Free PMC article. Review.
-
Review of 3D Cell Culture with Analysis in Microfluidic Systems.Anal Methods. 2019 Sep 7;11(33):4220-4232. doi: 10.1039/c9ay01328h. Epub 2019 Aug 6. Anal Methods. 2019. PMID: 32051693 Free PMC article.
-
3D-Printed Microfluidic Device with In-line Amperometric Detection that Also Enables Multi-Modal Detection.Anal Methods. 2020 Apr 21;12(15):2046-2051. doi: 10.1039/d0ay00368a. Epub 2020 Mar 27. Anal Methods. 2020. PMID: 32849919 Free PMC article.
-
Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices.Biosensors (Basel). 2020 Nov 19;10(11):182. doi: 10.3390/bios10110182. Biosensors (Basel). 2020. PMID: 33228050 Free PMC article. Review.
-
A Hybrid Nanofiber/Paper Cell Culture Platform for Building a 3D Blood-brain Barrier Model.Small Methods. 2021 Sep 15;5(9):2100592. doi: 10.1002/smtd.202100592. Epub 2021 Aug 16. Small Methods. 2021. PMID: 34541301 Free PMC article.
References
-
- Manz A, Harrison DJ, Verpoorte EMJ, Fettinger JC, Paulus A, Ludi H and Widmer HM, J Chromatogr A, 1992, 593, 253–258.
-
- Manz A, Graber N and Widmer HM, Sens Actuators, B, 1990, 1, 244–248.
-
- Harrison DJ, Manz A, Fan Z, Luedi H and Widmer HM, Anal Chem, 1992, 64, 1926–1932.
-
- Duffy DC, McDonald JC, Schueller OJA and Whitesides GM, Anal. Chem, 1998, 70, 4974–4984. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

