Supramolecular therapeutics to treat the side effects induced by a depolarizing neuromuscular blocking agent
- PMID: 31244944
- PMCID: PMC6567959
- DOI: 10.7150/thno.34947
Supramolecular therapeutics to treat the side effects induced by a depolarizing neuromuscular blocking agent
Abstract
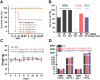
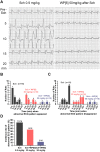
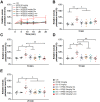
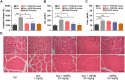
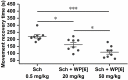
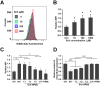
Succinylcholine (Sch) is the only depolarizing neuromuscular blocking agent widely used for rapid sequence induction in emergency rooms. Unfortunately, a variety of (sometimes lethal) adverse effects, such as hyperkalemia and cardiac arrest, are associated with its use, and currently there are no specific antidotes to reverse Sch or to treat these side-effects. Methods: The binding behaviors of Sch and several synthetic receptors, including cucurbit[7]uril, sulfo-calix[4]arene and water-soluble carboxylatopillar[6]arene (WP[6]), were first investigated. With a mouse model, a leathal dose of Sch was selected for evaluation of the antidotal effects of these synthetic receptors on Sch induced mortality. The antidotal effects of a selected synthetic receptor, WP[6], on Sch induced cardiac arrhythmias, hyperkalemia, rhabdomyolysis and paralysis were subsequently evaluated with rat and mouse models. The reversal mechanism was also investigated at a cellular level. Results: All of these macrocyclic molecules exhibited relatively high binding affinities with Sch in vitro. In a Sch-overdosed mouse model, immediate injection of these synthetic receptors right after Sch administration increased the overall survival rate, with WP[6] standing out with the most effective antidotal effects. In addition, administration of WP[6] also reversed the paralysis induced by Sch in a mouse model. Moreover, infusion of WP[6] to Sch-overdosed rats reduced the incidence of cardiac arrhythmia, inhibited the otherwise abnormally high serum potassium levels, and relieved the muscular damage. At the cellular level, WP[6] reversed the Sch induced depolarization and reduced the efflux of intracellular potassium. Conclusion: Synthetic receptors, particularly WP[6], exhibited high binding affinities towards Sch, and presented a significant potential as supramolecular therapeutics to treat the various side effects of Sch by specifically sequestering Sch in vivo.
Keywords: antidote; host-guest chemistry; neuromuscular blocking agent; pillararene; succinylcholine.
Conflict of interest statement
Competing Interests: X.Z. and R.W. are in the process of filing a patent relating to the content of this work. All other authors have no competing interest to declare.
Figures

Similar articles
-
Succinylcholine-induced hyperkalemia following prolonged pharmacologic neuromuscular blockade.Chest. 1997 Jan;111(1):248-50. doi: 10.1378/chest.111.1.248. Chest. 1997. PMID: 8996027
-
[Profile of the effect of succinylcholine after pre-curarization with atracurium, vecuronium or pancuronium].Anasthesiol Intensivmed Notfallmed Schmerzther. 1996 Jun;31(5):304-8. doi: 10.1055/s-2007-995925. Anasthesiol Intensivmed Notfallmed Schmerzther. 1996. PMID: 8767244 Clinical Trial. German.
-
Cardiac arrest from succinylcholine-induced hyperkalemia.Am J Health Syst Pharm. 2003 Apr 1;60(7):694-7. doi: 10.1093/ajhp/60.7.694. Am J Health Syst Pharm. 2003. PMID: 12701553 No abstract available.
-
[Heart arrest in children after intravenous injection of succinylcholine in the ENT operating room].HNO. 1995 Nov;43(11):676-9. HNO. 1995. PMID: 8530317 Review. German.
-
Cardiac arrest after succinylcholine: mortality greater with rhabdomyolysis than receptor upregulation.Anesthesiology. 2001 Mar;94(3):523-9. doi: 10.1097/00000542-200103000-00026. Anesthesiology. 2001. PMID: 11374616 Review. No abstract available.
Cited by
-
Flexible organic frameworks sequester neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo.Chem Sci. 2022 Jul 20;13(32):9243-9248. doi: 10.1039/d2sc02456j. eCollection 2022 Aug 17. Chem Sci. 2022. PMID: 36093029 Free PMC article.
-
Pillar[6]MaxQ: A Potent Supramolecular Host for In Vivo Sequestration of Methamphetamine and Fentanyl.Chem. 2023 Apr 13;9(4):881-900. doi: 10.1016/j.chempr.2022.11.019. Epub 2022 Dec 15. Chem. 2023. PMID: 37346394 Free PMC article.
-
Recent advances in supramolecular antidotes.Theranostics. 2021 Jan 1;11(3):1513-1526. doi: 10.7150/thno.53459. eCollection 2021. Theranostics. 2021. PMID: 33391548 Free PMC article. Review.
-
In Vitro and In Vivo Sequestration of Phencyclidine by Me4 Cucurbit[8]uril*.Chemistry. 2021 Feb 10;27(9):3098-3105. doi: 10.1002/chem.202004380. Epub 2021 Jan 19. Chemistry. 2021. PMID: 33206421 Free PMC article.
-
Supramolecular Partners: Precision On/Off Neuromuscular Blockage for Prolonged Surgeries.J Med Chem. 2025 Apr 10;68(7):6955-6957. doi: 10.1021/acs.jmedchem.5c00557. Epub 2025 Mar 6. J Med Chem. 2025. PMID: 40045846 Free PMC article.
References
-
- Appiah-Ankam J, Hunter JM. Pharmacology of neuromuscular blocking drugs. BJA Educ. 2004;4:2–7.
-
- Baraka A. Succinylcholine "the gold standard" for rapid-sequence induction of anesthesia. Middle East J Anesthesiol. 2011;21:323. - PubMed
-
- Al-alami AA, Zestos MM, Baraka AS. Pediatric laryngospasm: prevention and treatment. Curr Opin Anesthesiol. 2009;22:388–95. - PubMed
-
- Bryson EO, Aloysi AS, Popeo DM, Bodian CA, Pasculli RM, Briggs MC. et al. Methohexital and succinylcholine dosing for electroconvulsive therapy (ECT): actual versus ideal. J ECT. 2012;28:e29–e30. - PubMed
-
- Li EH, Bryson EO, Kellner CH. Muscle relaxation with succinylcholine in electroconvulsive therapy. Anesth Analg. 2016;123:1329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

