Metal-Organic Framework Nanoparticle-Based Biomineralization: A New Strategy toward Cancer Treatment
- PMID: 31244946
- PMCID: PMC6567975
- DOI: 10.7150/thno.33539
Metal-Organic Framework Nanoparticle-Based Biomineralization: A New Strategy toward Cancer Treatment
Abstract
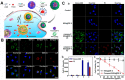
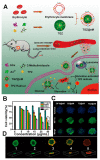
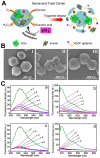
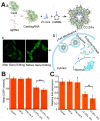
Cancer treatment using functional proteins, DNA/RNA, or complex bio-entities is important in both preclinical and clinical studies. With the help of nano-delivery systems, these biomacromolecules can enrich cancer tissues to match the clinical requirements. Biomineralization via a self-assembly process has been widely applied to provide biomacromolecules exoskeletal-like protection for immune shielding and preservation of bioactivity. Advanced metal-organic framework nanoparticles (MOFs) are excellent supporting matrices due to the low toxicity of polycarboxylic acids and metals, high encapsulation efficiency, and moderate synthetic conditions. In this review, we study MOFs-based biomineralization for cancer treatment and summarize the unique properties of MOF hybrids. We also evaluate the outlook of potential cancer treatment applications for MOFs-based biomineralization. This strategy likely opens new research orientations for cancer theranostics.
Keywords: biomineralization; cancer treatment; metal-organic framework; theranostics.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Bioengineering of Metal-organic Frameworks for Nanomedicine.Theranostics. 2019 May 18;9(11):3122-3133. doi: 10.7150/thno.31918. eCollection 2019. Theranostics. 2019. PMID: 31244945 Free PMC article. Review.
-
Recent Developments of Supramolecular Metal-based Structures for Applications in Cancer Therapy and Imaging.Theranostics. 2019 May 18;9(11):3150-3169. doi: 10.7150/thno.31828. eCollection 2019. Theranostics. 2019. PMID: 31244947 Free PMC article. Review.
-
Polyphenol-Based Particles for Theranostics.Theranostics. 2019 May 18;9(11):3170-3190. doi: 10.7150/thno.31847. eCollection 2019. Theranostics. 2019. PMID: 31244948 Free PMC article. Review.
-
Theranostics of metal-organic frameworks: image-guided nanomedicine for clinical translation.Nanomedicine (Lond). 2023 Apr;18(8):695-703. doi: 10.2217/nnm-2022-0278. Epub 2023 Jun 1. Nanomedicine (Lond). 2023. PMID: 37259854 Review.
-
Biomineralization: An Opportunity and Challenge of Nanoparticle Drug Delivery Systems for Cancer Therapy.Adv Healthc Mater. 2020 Nov;9(22):e2001117. doi: 10.1002/adhm.202001117. Epub 2020 Oct 11. Adv Healthc Mater. 2020. PMID: 33043640 Review.
Cited by
-
Synthesis and evaluation of gene delivery vectors based on PEI-modified metal-organic framework (MOF) nanoparticles.Iran J Basic Med Sci. 2024;27(2):203-213. doi: 10.22038/IJBMS.2023.71892.15644. Iran J Basic Med Sci. 2024. PMID: 38234668 Free PMC article.
-
Cyclodextrin-Based Metal-Organic Framework as an Application Platform for Bioactive Ruthenium(III) Complexes.Inorg Chem. 2025 Jun 9;64(22):10870-10878. doi: 10.1021/acs.inorgchem.5c00813. Epub 2025 May 23. Inorg Chem. 2025. PMID: 40408128 Free PMC article.
-
Metal-Organic Frameworks: A Potential Platform for Enzyme Immobilization and Related Applications.Front Bioeng Biotechnol. 2020 Jun 30;8:695. doi: 10.3389/fbioe.2020.00695. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32695766 Free PMC article. Review.
-
Applications of nanocomposites based on zeolitic imidazolate framework-8 in photodynamic and synergistic anti-tumor therapy.RSC Adv. 2022 Jun 9;12(26):16927-16941. doi: 10.1039/d2ra01102f. eCollection 2022 Jun 1. RSC Adv. 2022. PMID: 35754870 Free PMC article. Review.
-
Fabrication of Porous Fe-Based Metal-Organic Complex for the Enhanced Delivery of 5-Fluorouracil in In Vitro Treatment of Cancer Cells.ACS Omega. 2022 Dec 9;7(50):46674-46681. doi: 10.1021/acsomega.2c05614. eCollection 2022 Dec 20. ACS Omega. 2022. PMID: 36570299 Free PMC article.
References
-
- Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional Nanomaterials for Phototherapies of Cancer. Chem Rev. 2014;114:10869–939. - PubMed
-
- Fan W, Yung B, Huang P, Chen X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem Rev. 2017;117:13566–638. - PubMed
-
- Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016;16:2334–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

