Current options and future possibilities for the systemic treatment of hepatocellular carcinoma
- PMID: 31244990
- PMCID: PMC6571544
- DOI: 10.2217/hep-2019-0001
Current options and future possibilities for the systemic treatment of hepatocellular carcinoma
Abstract
Most hepatocellular carcinoma patients could not benefit from or experience disease recurrence after curative treatments. In 2007 sorafenib demonstrated efficacy in first line treatment of advanced hepatocellular carcinoma. After a decade of negative trials, in early 2019 we now have another tyrosine kinase inhibitor available in first line, lenvatinib, three other targeted therapies in second line post-sorafenib (regorafenib, cabozantinib and ramucirumab) and promising data from two immunotherapies (nivolumab and pembrolizumab). Unfortunately, no biomarkers have been identified to help guide our choice. In this short review we summarize the results of these different therapies and propose a therapeutic algorithm based on subgroup analysis. It is most likely that we will not have head-to-head comparisons in second line trials.
Keywords: cabozantinib; lenvatinib; nivolumab; pembrolizumab; ramucirumab; regorafenib; sorafenib.
Conflict of interest statement
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Figures
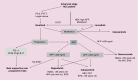
References
-
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol. 69(1), 182–236 (2018). - PubMed
-
•• More recent guidelines.
-
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 10(11), 656–665 (2013). - PubMed
-
- Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S. et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 3(12), 1683–1691 (2017). - PMC - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 391(10127), 1301–1314 (2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
