Era of biosimilars in rheumatology: reshaping the healthcare environment
- PMID: 31245050
- PMCID: PMC6560670
- DOI: 10.1136/rmdopen-2019-000900
Era of biosimilars in rheumatology: reshaping the healthcare environment
Abstract
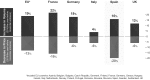
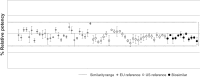
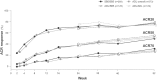
Compared with the original approved biological drug on which it is based, a biosimilar has highly similar physicochemical characteristics and biological activity, as well as equivalent efficacy and no clinically meaningful differences in safety and immunogenicity. Before they are approved, biosimilars must undergo a rigorous development process using state-of-the-art technologies to establish biosimilarity to the reference biological product. After approval, biosimilars must comply with good pharmacological practices for biological drugs. Several biosimilar disease-modifying antirheumatic drugs (bsDMARDs) based on the tumour necrosis factor inhibitors adalimumab, etanercept and infliximab have been approved for use in patients with rheumatic diseases. Substantial cost savings can be made if biological-naive patients begin treatment with bsDMARDs, and patients receiving original biological DMARDs (bDMARDs) are switched to bsDMARDs. Despite the consistently similar efficacy, safety and immunogenicity of bsDMARDs relative to their respective original bDMARDs, switching from a reference bDMARD to a bsDMARD can result in nocebo responses, such as subjective increase of disease activity and pain-related adverse events. This may have a negative impact on adherence to bsDMARDs in clinical trials and clinical practice. To ensure optimal and rational integration of bsDMARDs into rheumatology practice and realise the full cost-saving efficacy of these drugs, rheumatologists must be aware that careful communication of the cost-saving efficacy and safety of bsDMARDs to their patients is the key to a successful long-term switch to bsDMARD therapy.
Keywords: anti-tnf; arthritis; autoimmune diseases; dmards (biologic); rheumatoid arthritis.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
