Prospective analysis of 895 patients on a UK Genomics Review Board
- PMID: 31245058
- PMCID: PMC6557082
- DOI: 10.1136/esmoopen-2018-000469
Prospective analysis of 895 patients on a UK Genomics Review Board
Abstract
Background: The increasing frequency and complexity of cancer genomic profiling represents a challenge for the oncology community. Results from next-generation sequencing-based clinical tests require expert review to determine their clinical relevance and to ensure patients are stratified appropriately to established therapies or clinical trials.
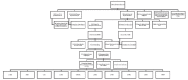
Methods: The Sarah Cannon Research Institute UK/UCL Genomics Review Board (GRB) was established in 2014 and represents a multidisciplinary team with expertise in molecular oncology, clinical trials, clinical cancer genetics and molecular pathology. Prospective data from this board were collated.
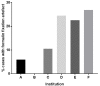
Results: To date, 895 patients have been reviewed by the GRB, of whom 180 (20%) were referred for clinical trial screening and 62 (7%) received trial therapy. For a further 106, a clinical trial recommendation was given.
Conclusions: Numerous challenges are faced in implementing a GRB, including the identification of potential germline variants, the interpretation of variants of uncertain significance and consideration of the technical limitations of pathology material when interpreting results. These challenges are likely to be encountered with increasing frequency in routine practice. This GRB experience provides a model for the multidisciplinary review of molecular profiling data and for the linking of molecular analysis to clinical trial networks.
Keywords: clinical genetics; genomic medicine; molecular oncology; molecular tumour board.
Conflict of interest statement
Competing interests: PB has participated in ThermoFisher’s European Clinical Oncology Advisory Board meetings, and has delivered sponsored, but not for profit, presentations at the invitation of ThermoFisher UK on a number of occasions. CS reports grant support from Cancer Research UK, UCLH Biomedical Research Council, Rosetrees Trust and AstraZeneca. Personal fees from Boehringer Ingelheim, Novartis, Eli Lilly, Roche Ventana, GlaxoSmithKline, Pfizer, Genentech and Celgene. Stock options in GRAIL, APOGEN Biotechnologies and EPIC Bioscience and has stock options and is cofounder of Achilles Therapeutics.
Figures


Comment on
-
Advancing molecular tumour boards: highly needed to maximise the impact of precision medicine.ESMO Open. 2019 Apr 20;4(2):e000516. doi: 10.1136/esmoopen-2019-000516. eCollection 2019. ESMO Open. 2019. PMID: 31233036 Free PMC article. No abstract available.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

