Cisplatin Eligibility Issues and Alternative Regimens in Locoregionally Advanced Head and Neck Cancer: Recommendations for Clinical Practice
- PMID: 31245288
- PMCID: PMC6579895
- DOI: 10.3389/fonc.2019.00464
Cisplatin Eligibility Issues and Alternative Regimens in Locoregionally Advanced Head and Neck Cancer: Recommendations for Clinical Practice
Abstract
Well-designed randomized trials provide the highest level of scientific evidence to guide clinical decision making. In chemoradiotherapy of locally advanced squamous cell carcinoma of the head and neck (SCCHN), data support the use of three cycles of 100 mg/m2 cisplatin given every 3 weeks concurrently with conventionally fractionated external beam radiotherapy, although a full compliance with all three cycles is reserved to only about two thirds of initially eligible cases. On an individual patient level, practicing oncologists have to determine whether the patient is a suitable candidate for this treatment or whether contraindications exist. In the latter case, an adequate alternative has to be offered. In this regard, to facilitate triaging of medical information, we reviewed available publications on this topic and prepared practice-oriented recommendations for systemic treatment concurrent to definitive and post-operative radiotherapy. Even if no contraindications for the standard-of-care cisplatin apply, clinicians may opt for alternative regimens by adjusting the peak dose, cumulative dose, or timing of cisplatin. Relative contraindications pose the major issue in clinical practice, as very limited data is available in the literature and final decisions are usually based on an expert opinion or retrospective cohort studies. In the case of absolute interdiction of cisplatin, several alternative regimens incorporating carboplatin, 5-fluorouracil, cetuximab, and docetaxel are available. At the same time, it should be kept in mind that radiotherapy alone represents a viable option with hyperfractionation being particularly beneficial in the definitive management of limited nodal disease. Ideally, all treatment propositions should be discussed within multidisciplinary tumor boards taking into account the patient- and disease-related characteristics as well as local logistics and reimbursement policies.
Keywords: cetuximab; chemoradiotherapy; cisplatin; clinical trials; head and neck cancer; immunotherapy; practice recommendations; targeted therapy.
Figures
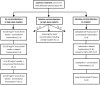

References
-
- Vermorken JB. Patient and treatment factors in concurrent chemoradiotherapy. In: Vermorken JB, Budach V, Leemans CR, Machiels JP, Nicolai P, O'Sullivan B. editors. Critical Issues in Head and Neck Oncology: Key Concepts From the Fifth THNO Meeting. Cham: Springer International Publishing AG; (2017). p. 189–201.
-
- Adelstein DJ, Li Y, Adams GL, Wagner H Jr., Kish JA, Ensley JF, et al. . An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. (2003) 21:92–8. 10.1200/JCO.2003.01.008 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

