Pediatric Mesenchymal Stem Cells Exhibit Immunomodulatory Properties Toward Allogeneic T and B Cells Under Inflammatory Conditions
- PMID: 31245368
- PMCID: PMC6581756
- DOI: 10.3389/fbioe.2019.00142
Pediatric Mesenchymal Stem Cells Exhibit Immunomodulatory Properties Toward Allogeneic T and B Cells Under Inflammatory Conditions
Abstract
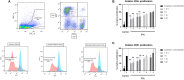
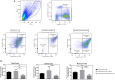
Mesenchymal stem cells from pediatric patients (pMSCs) are an attractive cell source in regenerative medicine, due to their higher proliferation rates and better differentiation abilities compared to adult MSCs (aMSCs). We have previously characterized the immunomodulatory abilities of pMSCs on T cells under co-culture. It has also been reported that aMSCs can inhibit B cell proliferation and maturation under inflammatory conditions. In this study, we therefore aimed to clarify the immunomodulatory effect of pMSCs toward T and B cells in an inflammatory microenvironment. Bone marrow derived pMSCs were primed to simulate inflammatory conditions by exposure with 50 ng/mL of IFN-γ for 3 days. To analyze the interaction between pMSCs and T cells, CD3/CD28 stimulated peripheral blood mononuclear cells (PBMCs) were co-cultured with primed or unprimed pMSCs. To investigate B cell responses, quiescent B cells obtained from spleens by CD43 negative selection were stimulated with anti-IgM, anti-CD40, IL-2, and co-cultured with either IFN-γ primed or unprimed pMSC. pMSC phenotype, B and T cell proliferation, and B cell functionality were analyzed. Gene expression of indoleamine 2,3-dioxygenease (IDO), as well as the expression of HLA-ABC, HLA-DR and the co-stimulatory molecules CD80 and CD86 was upregulated on pMSCs upon IFN-γ priming. IFN-γ did not alter the immunomodulatory abilities of pMSCs upon CD4+ nor CD8+ stimulated T cells compared to unprimed pMSCs. IFN-γ primed pMSCs but not unprimed pMSCs strongly inhibited naïve (CD19+CD27-), memory (CD19+CD27+), and total B cell proliferation. Antibody-producing plasmablast (CD19+CD27highCD38high) formation and IgG production were also significantly inhibited by IFN-γ primed pMSCs compared to unprimed pMSCs. Collectively, these results show that pMSCs have immunomodulatory effects upon the adaptive immune response which can be potentiated by inflammatory stimuli. This knowledge is useful in regenerative medicine and allogeneic transplantation applications toward tailoring pMSCs function to best modulate the immune response for a successful implant engraftment and avoidance of a strong immune reaction.
Keywords: B cell; T cell; allogeneic; immunomodulation; inflammatory microenvironment; mesenchymal stem cell.
Figures

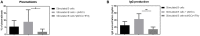
Similar articles
-
Human Chorionic Villous Mesenchymal Stem Cells Modify the Functions of Human Dendritic Cells, and Induce an Anti-Inflammatory Phenotype in CD1+ Dendritic Cells.Stem Cell Rev Rep. 2015 Jun;11(3):423-41. doi: 10.1007/s12015-014-9562-8. Stem Cell Rev Rep. 2015. PMID: 25287760
-
Placenta-derived multipotent mesenchymal stromal cells: a promising potential cell-based therapy for canine inflammatory brain disease.Stem Cell Res Ther. 2020 Jul 22;11(1):304. doi: 10.1186/s13287-020-01799-0. Stem Cell Res Ther. 2020. PMID: 32698861 Free PMC article.
-
Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function.Front Immunol. 2017 Aug 28;8:1042. doi: 10.3389/fimmu.2017.01042. eCollection 2017. Front Immunol. 2017. PMID: 28894451 Free PMC article.
-
The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation.Stem Cell Rev Rep. 2014 Feb;10(1):79-85. doi: 10.1007/s12015-013-9478-8. Stem Cell Rev Rep. 2014. PMID: 24142483 Free PMC article. Review.
-
Mesenchymal stem cells derived from hard palate: An attractive alternative for regenerative medicine.Oral Dis. 2024 Oct;30(7):4145-4151. doi: 10.1111/odi.15043. Epub 2024 Jul 15. Oral Dis. 2024. PMID: 39007203 Review.
Cited by
-
Role of the CXCR4-SDF1-HMGB1 pathway in the directional migration of cells and regeneration of affected organs.World J Stem Cells. 2020 Sep 26;12(9):938-951. doi: 10.4252/wjsc.v12.i9.938. World J Stem Cells. 2020. PMID: 33033556 Free PMC article. Review.
-
The Effect of Cell Culture Passage on the Efficacy of Mesenchymal Stromal Cells as a Cell Therapy Treatment.J Clin Med. 2024 Apr 24;13(9):2480. doi: 10.3390/jcm13092480. J Clin Med. 2024. PMID: 38731011 Free PMC article.
-
Operative Management of Avascular Necrosis of the Femoral Head in Skeletally Immature Patients: A Systematic Review.Life (Basel). 2022 Jan 26;12(2):179. doi: 10.3390/life12020179. Life (Basel). 2022. PMID: 35207467 Free PMC article. Review.
-
Mesenchymal Stem Cells Isolated from Paediatric Paravertebral Adipose Tissue Show Strong Osteogenic Potential.Biomedicines. 2022 Feb 4;10(2):378. doi: 10.3390/biomedicines10020378. Biomedicines. 2022. PMID: 35203587 Free PMC article.
-
Chondrogenically Primed Human Mesenchymal Stem Cells Persist and Undergo Early Stages of Endochondral Ossification in an Immunocompetent Xenogeneic Model.Front Immunol. 2021 Sep 30;12:715267. doi: 10.3389/fimmu.2021.715267. eCollection 2021. Front Immunol. 2021. PMID: 34659205 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

