Rewiring the Regenerated Zebrafish Retina: Reemergence of Bipolar Neurons and Cone-Bipolar Circuitry Following an Inner Retinal Lesion
- PMID: 31245369
- PMCID: PMC6562337
- DOI: 10.3389/fcell.2019.00095
Rewiring the Regenerated Zebrafish Retina: Reemergence of Bipolar Neurons and Cone-Bipolar Circuitry Following an Inner Retinal Lesion
Abstract
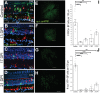
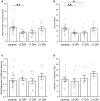
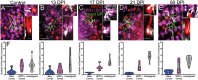
We previously reported strikingly normal morphologies and functional connectivities of regenerated retinal bipolar neurons (BPs) in zebrafish retinas sampled 60 days after a ouabain-mediated lesion of inner retinal neurons (60 DPI) (McGinn et al., 2018). Here we report early steps in the birth of BPs and formation of their dendritic trees and axonal arbors during regeneration. Adult zebrafish were subjected to ouabain-mediated lesion that destroys inner retinal neurons but spares photoreceptors and Müller glia, and were sampled at 13, 17, and 21 DPI, a timeframe over which plexiform layers reemerge. We show that this timeframe corresponds to reemergence of two populations of BPs (PKCα+ and nyx::mYFP+). Sequential BrdU, EdU incorporation reveals that similar fractions of PKCα+ BPs and HuC/D+ amacrine/ganglion cells are regenerated concurrently, suggesting that the sequence of neuronal production during retinal regeneration does not strictly match that observed during embryonic development. Further, accumulation of regenerated BPs appears protracted, at least through 21 DPI. The existence of isolated, nyx::mYFP+ BPs allowed examination of cytological detail through confocal microscopy, image tracing, morphometric analyses, identification of cone synaptic contacts, and rendering/visualization. Apically-projecting neurites (=dendrites) of regenerated BPs sampled at 13, 17, and 21 DPI are either truncated, or display smaller dendritic trees when compared to controls. In cases where BP dendrites reach the outer plexiform layer (OPL), numbers of dendritic tips are similar to those of controls at all sampling times. Further, by 13-17 DPI, BPs with dendritic tips reaching the outer nuclear layer (ONL) show patterns of photoreceptor connections that are statistically indistinguishable from controls, while those sampled at 21 DPI slightly favor contacts with double cone synaptic terminals over those of blue-sensitive cones. These findings suggest that once regenerated BP dendrites reach the OPL, normal photoreceptor connectomes are established, albeit with some plasticity. Through 17 DPI, some basally-projecting neurites (=axons) of regenerated nyx::mYFP+ BPs traverse long distances, branch into inappropriate layers, or appear to abruptly terminate. These findings suggest that, after a tissue-disrupting lesion, regeneration of inner retinal neurons is a dynamic process that includes ongoing genesis of new neurons and changes in BP morphology.
Keywords: birthdating; circuitry; connectome; photoreceptor; regeneration; retina; retinal bipolar cell; zebrafish.
Figures






Similar articles
-
Restoration of Dendritic Complexity, Functional Connectivity, and Diversity of Regenerated Retinal Bipolar Neurons in Adult Zebrafish.J Neurosci. 2018 Jan 3;38(1):120-136. doi: 10.1523/JNEUROSCI.3444-16.2017. Epub 2017 Nov 13. J Neurosci. 2018. PMID: 29133431 Free PMC article.
-
Assessing Rewiring of the Retinal Circuitry by Electroretinogram (ERG) After Inner Retinal Lesion in Adult Zebrafish.Methods Mol Biol. 2023;2636:421-435. doi: 10.1007/978-1-0716-3012-9_23. Methods Mol Biol. 2023. PMID: 36881314 Free PMC article.
-
Dynamic functional and structural remodeling during retinal regeneration in zebrafish.Front Mol Neurosci. 2022 Nov 30;15:1070509. doi: 10.3389/fnmol.2022.1070509. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36533135 Free PMC article.
-
Neural remodeling in retinal degeneration.Prog Retin Eye Res. 2003 Sep;22(5):607-55. doi: 10.1016/s1350-9462(03)00039-9. Prog Retin Eye Res. 2003. PMID: 12892644 Review.
-
Evidence of regional specializations in regenerated zebrafish retina.Exp Eye Res. 2021 Nov;212:108789. doi: 10.1016/j.exer.2021.108789. Epub 2021 Oct 13. Exp Eye Res. 2021. PMID: 34653519 Free PMC article. Review.
Cited by
-
Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina.Cells. 2021 Mar 12;10(3):633. doi: 10.3390/cells10030633. Cells. 2021. PMID: 33809186 Free PMC article. Review.
-
The Healthy and Diseased Retina Seen through Neuron-Glia Interactions.Int J Mol Sci. 2024 Jan 17;25(2):1120. doi: 10.3390/ijms25021120. Int J Mol Sci. 2024. PMID: 38256192 Free PMC article. Review.
-
Visual Function is Gradually Restored During Retina Regeneration in Adult Zebrafish.Front Cell Dev Biol. 2022 Feb 1;9:831322. doi: 10.3389/fcell.2021.831322. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35178408 Free PMC article.
-
Development and characterization of a chronic photoreceptor degeneration model in adult zebrafish that does not trigger a regenerative response.Exp Eye Res. 2021 Aug;209:108630. doi: 10.1016/j.exer.2021.108630. Epub 2021 May 21. Exp Eye Res. 2021. PMID: 34029596 Free PMC article.
-
Synaptic or Non-synaptic? Different Intercellular Interactions with Retinal Ganglion Cells in Optic Nerve Regeneration.Mol Neurobiol. 2022 May;59(5):3052-3072. doi: 10.1007/s12035-022-02781-y. Epub 2022 Mar 9. Mol Neurobiol. 2022. PMID: 35266115 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

