The Neural Crest as the First Production Site of the Erythroid Growth Factor Erythropoietin
- PMID: 31245372
- PMCID: PMC6581680
- DOI: 10.3389/fcell.2019.00105
The Neural Crest as the First Production Site of the Erythroid Growth Factor Erythropoietin
Abstract
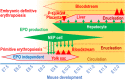
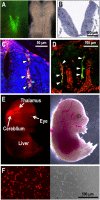
While the neural crest is considered the fourth germ layer that originates a variety of tissues during mammalian development, we recently discovered that some neural crest cells and neuroepithelial cells play a unique role in secreting a vital hematopoietic hormone, erythropoietin (EPO), in mouse embryos. EPO production by the neural crest is transient in mid-stage embryos but essential for the first erythropoiesis in the yolk sac and for sufficient oxygen supply in the whole embryo growing in utero. The site of EPO production shifts from the neural crest to the liver in late embryonic stages, followed by interstitial fibroblasts of the kidneys in adults. Interestingly, the transition of EPO production sites synchronizes with the transition of erythropoietic sites during mouse development from the yolk sac and the fetal liver to the bone marrow. EPO produced by the neural crest and the neuroepithelium is first stored around the production sites and delivered to the yolk sac as an endocrine hormone for erythropoiesis after heartbeat activation. The fact that EPO is produced by some human cell lines derived from neuroblastoma, which mainly originates from the neural crest, provides evidence that the neural crest secretes EPO for primitive erythropoiesis not only in mouse but also in human embryos. Here, we introduce and discuss recent progress in studies on the mechanism of EPO production by the neural crest and its roles in erythropoietic development and in the fate of EPO-producing neural crest cells.
Keywords: REP cell; erythropoiesis; erythropoietin; hypoxia; reporter mice.
Figures
Similar articles
-
Erythropoietin production in neuroepithelial and neural crest cells during primitive erythropoiesis.Nat Commun. 2013;4:2902. doi: 10.1038/ncomms3902. Nat Commun. 2013. PMID: 24309470
-
Erythropoietin gene expression: developmental-stage specificity, cell-type specificity, and hypoxia inducibility.Tohoku J Exp Med. 2015 Mar;235(3):233-40. doi: 10.1620/tjem.235.233. Tohoku J Exp Med. 2015. PMID: 25786542 Review.
-
Hypoxia Signaling in the Cell Type-Specific Regulation of Erythropoietin Production Throughout Mammalian Development.Mol Cell Biol. 2025;45(9):386-394. doi: 10.1080/10985549.2025.2522720. Epub 2025 Jun 27. Mol Cell Biol. 2025. PMID: 40574673 Review.
-
Erythropoietin Production in Embryonic Neural Cells is Controlled by Hypoxia Signaling and Histone Deacetylases with an Undifferentiated Cellular State.Mol Cell Biol. 2025 Jan;45(1):32-45. doi: 10.1080/10985549.2024.2428717. Epub 2024 Dec 2. Mol Cell Biol. 2025. PMID: 39620278
-
Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development.J Biol Chem. 2006 Sep 1;281(35):25703-11. doi: 10.1074/jbc.M602329200. Epub 2006 Jun 19. J Biol Chem. 2006. PMID: 16787915
Cited by
-
The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization.Int J Mol Sci. 2021 Jul 19;22(14):7682. doi: 10.3390/ijms22147682. Int J Mol Sci. 2021. PMID: 34299300 Free PMC article. Review.
-
Signaling Pathways That Regulate Normal and Aberrant Red Blood Cell Development.Genes (Basel). 2021 Oct 19;12(10):1646. doi: 10.3390/genes12101646. Genes (Basel). 2021. PMID: 34681039 Free PMC article. Review.
-
The Many Facets of Erythropoietin Physiologic and Metabolic Response.Front Physiol. 2020 Jan 17;10:1534. doi: 10.3389/fphys.2019.01534. eCollection 2019. Front Physiol. 2020. PMID: 32038269 Free PMC article. Review.
-
Yolk sac cell atlas reveals multiorgan functions during human early development.Science. 2023 Aug 18;381(6659):eadd7564. doi: 10.1126/science.add7564. Epub 2023 Aug 18. Science. 2023. PMID: 37590359 Free PMC article.
-
Alteration of the DNA Methylation Signature of Renal Erythropoietin-Producing Cells Governs the Sensitivity to Drugs Targeting the Hypoxia-Response Pathway in Kidney Disease Progression.Front Genet. 2019 Nov 13;10:1134. doi: 10.3389/fgene.2019.01134. eCollection 2019. Front Genet. 2019. PMID: 31798631 Free PMC article. Review.
References
-
- George L., Dunkel H., Hunnicutt B. J., Filla M., Little C., Lansford R., et al. (2016). In vivo time-lapse imaging reveals extensive neural crest and endothelial cell interactions during neural crest migration and formation of the dorsal root and sympathetic ganglia. Dev. Biol. 413 70–85. 10.1016/j.ydbio.2016.02.028 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

